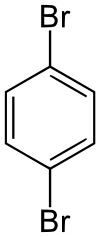
1,4-Dibromobenzene
1,4-Dibromobenzene (p-dibromobenzene) is an organic compound that is solid at room temperature. This compound has two bromine atoms (bromo substituents) off the central benzene ring. It has a strong smell similar to that of the lighter chlorine analogue. It can be used as a precursor to the dye 6,6-Dibromoindigo.[4]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,4-Dibromobenzene | |
| Other names
p-Dibromobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.083 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H4Br2 | |
| Molar mass | 235.906 g·mol−1 |
| Appearance | White solid |
| Density | 1.84 g/cm3 [1] |
| Melting point | 87 °C (189 °F; 360 K)[2] |
| Boiling point | 220.4 °C (428.7 °F; 493.5 K)[2] |
| Practically insoluble[3] | |
| Solubility in other solvents | Soluble in 70 parts ethanol[3] Soluble in benzene, chloroform and very soluble in diethyl ether[3] |
| -101.4·10−6 cm3/mol | |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Warning |
| H315, H319, H335, H400, H411 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Related compounds
- 1,2-Dibromobenzene
- 1,3-Dibromobenzene
References
- "Safety data for 1,4-dibromobenzene". Retrieved 23 November 2011.
- "1,4-Dibromobenzene LS026". Archived from the original on 2011-07-11. Retrieved 23 November 2011.
- Merck Index (14th ed.). Whitehouse Station, NJ: Merck & Co Inc. 2006. p. 3024.
- Wolk, Joel; Frimer, Aryeh (29 Nov 2020). "A Simple, Safe and Efficient Synthesis of Tyrian Purple (6,6-Dibromoindigo)". Molecules. doi:10.3390/molecules15085561 – via PubMed Central.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.