2,3-Dihydrothiophene
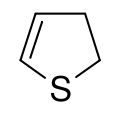
2,3-Dihydrothiophene is a heterocyclic compound and an organosulfur compound with the formula SC4H6. It is isomeric with the more symmetrical 2,5-dihydrothiophene. Both isomers of dihydrothiophene are colorless liquids with a thioether-like odor. In terms of their reactivity, both isomers exhibit characteristics of alkenes and thioethers, undergoing addition reactions at carbon and oxidation at sulfur. In contrast, thiophene engages in neither reaction.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2,3-Dihydrothiophene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6S | |
| Molar mass | 86.16 g/mol |
| Appearance | colorless liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Dihydrothiophenes in nature
Dihydrothiophenes contribute to the aroma of the white truffle. The major component is 3-methyl-4,5-dihydrothiophene (alternative name:4-methyl-2,3-dihydrothiophene), produced by bacterial colonies in the truffle's fruiting bodies.[2]
References
- Shvekhgeimer, M. G. A. (1998). "Dihydrothiophenes. Synthesis and Properties (review)". Chemistry of Heterocyclic Compounds. 34: 1101–1122. doi:10.1007/BF02319487.
- Splivallo, R.; Ebeler, S. E. (2015). "Sulfur Volatiles of microbial origin are key contributors to human-sensed truffle aroma". Appl. Microbiol. Biotechnol. 99: 2583–92. doi:10.1007/s00253-014-6360-9. PMID 25573471.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.