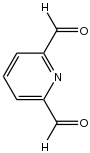
2,6-Diformylpyridine
2,6-Diformylpyridine is an organic compound with the formula C5H3N(CHO)2. The molecule features formyl groups adjacent to the nitrogen of pyridine. The compound is prepared by oxidation of 2,6-dimethylpyridine.[1]
 | |
| Names | |
|---|---|
| IUPAC name
pyridine-2,6-dicarbaldehyde | |
| Other names
2,6-Pyridinedialdehyde | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.024.172 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Appearance | white solid |
| Melting point | 124 °C (255 °F; 397 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It condenses with amines to give diiminopyridine ligands.[2][3]
References
- Forni, Lucio; Casalone, Gianluigi (1987). "Vapour Phase Oxidation of 2,6-Lutidine to 2,6-Pyridinedicarboxaldehyde. III: Kinetic Study". Applied Catalysis. 34: 317–328. doi:10.1016/S0166-9834(00)82465-3.
- Britovsek, George J. P.; Bruce, Michael; Gibson, Vernon C.; Kimberley, Brian S.; Maddox, Peter J.; Mastroianni, Sergio; McTavish, Stuart J.; Redshaw, Carl; Solan, Gregory A.; Strömberg, Staffan; White, Andrew J. P.; Williams, David J. (1999). "Iron and Cobalt Ethylene Polymerization Catalysts Bearing 2,6-Bis(Imino)Pyridyl Ligands: Synthesis, Structures, and Polymerization Studies". Journal of the American Chemical Society. 121 (38): 8728–8740. doi:10.1021/ja990449w.
- Chichak, K. S. (2004). "Molecular Borromean Rings" (PDF). Science. 304 (5675): 1308–1312. doi:10.1126/science.1096914. PMID 15166376. S2CID 45191675.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.