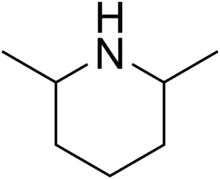
2,6-Dimethylpiperidine
2,6-Dimethylpiperidines are chemical compounds with the formula C5H8(CH3)2NH. Three stereoisomers exist: the achiral (R,S)-isomer and the chiral (R,R)/(S,S) enantiomeric pair. Dimethylpiperidines are derivatives of the heterocycle piperidine, wherein two hydrogen atoms are replaced by methyl groups.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2,6-Dimethylpiperidine | |
| Other names
2,6-Lupetidine; nanofin; nanophyn | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.256 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H15N | |
| Molar mass | 113.204 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.84 g/mL |
| Boiling point | 113.2 °C (235.8 °F; 386.3 K) |
| Low | |
| Solubility in other solvents | Most organic solvents |
| Basicity (pKb) | ca. 10 |
Refractive index (nD) |
1.4394 |
| Hazards | |
| Main hazards | Flammable |
| GHS pictograms |    |
| GHS Signal word | Danger |
| H225, H314, H315, H319, H335 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P271, P280, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P312, P321, P332+313, P337+313, P362, P363, P370+378, P403+233 | |
| Flash point | 11 °C (52 °F; 284 K) |
| Related compounds | |
Related compounds |
Piperidine |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The 2,6-dimethylpiperidines are prepared by reduction of 2,6-dimethylpyridine (2,6-lutidine). The achiral isomer is the predominant isomer produced in this reaction.
The 2,6-dimethylpiperidines are of interest for their conformational properties. The (R,S)-isomer exists largely in the chair conformation with equatorial methyl groups. The (R,R)/(S,S)-isomers are attractive chiral secondary amine building blocks.[1]
References
- Einhorn, J.; Einhorn, C.; Ratajczak, F.; Durif, A.; Averbuch, M.-T. and Pierre, J.-L., "Synthesis and resolution of a chiral analog of 2,2,6,6-tetramethylpiperidine and of its corresponding nitroxide", Tetrahedron Letters, 1998, volume 39, pages 2565-2568
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.