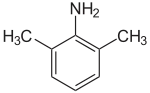
2,6-Xylidine
2,6-Xylidine is an organic compound with the formula C6H3(CH3)2NH2. It is one of several isomeric xylidines. It is a colorless viscous liquid. Commercially significant derivatives are the anesthetics lidocaine, bupivacaine, mepivacaine, and etidocaine.[1]
 | |
| Names | |
|---|---|
| IUPAC name
2,6-Dimethylaniline | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.599 |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H11N | |
| Molar mass | 121.183 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9842 g/mL |
| Melting point | 11.45 °C (52.61 °F; 284.60 K) |
| Boiling point | 215 °C (419 °F; 488 K) |
Refractive index (nD) |
1.5601 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Production and reactions
Many xylidines are prepared by nitration of a xylene followed by hydrogenation of the nitroaromatic, but this approach is not efficient for this isomer. Instead, it is prepared from by treatment of the related xylenol with ammonia in the presence of oxide catalysts.[1]
References
- M. Meyer (2012). "Xylidines". Ullmann's Encylclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_455.
- Ison, Elon A.; Ison, Ana (2012). "Synthesis of Well-Defined CopperN-Heterocyclic Carbene Complexes and Their Use as Catalysts for a "Click Reaction": A Multistep Experiment That Emphasizes the Role of Catalysis in Green Chemistry". Journal of Chemical Education. 89 (12): 1575. Bibcode:2012JChEd..89.1575I. doi:10.1021/ed300243s.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.