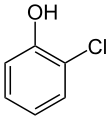
2-Chlorophenol
2-Chlorophenol or ortho-chlorophenol is an organic compound with the formula C6H4Cl(OH). It is one of three isomeric monochloride derivatives of phenol. As from occasional use as a disinfectant, it has few applications. It is an intermediate in the polychlorination of phenol.[6] 2-Chlorophenol is a colorless liquid, although commercial samples are often yellow or amber-colored. It has an unpleasant, penetrating (carbolic) odor. It is poorly soluble in water.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
2-Chlorophenol[5] | |||
| Other names
o-Chlorophenol ortho-Chlorophenol 2-Hydroxychlorobenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.002.213 | ||
| KEGG | |||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H5ClO | |||
| Molar mass | 128.56 g·mol−1 | ||
| Appearance | colorless liquid | ||
| Density | 1.2634 g/cm3 at 20 °C | ||
| Melting point | 9.4 °C (48.9 °F; 282.5 K) | ||
| Boiling point | 174.9 °C (346.8 °F; 448.0 K) | ||
| 20 g/L at 20 °C | |||
| Solubility | Soluble in ethanol, diethyl ether, benzene | ||
| Vapor pressure | 0.308 kPa | ||
| Acidity (pKa) | 8.56 | ||
| -77.4·10−6 cm3/mol | |||
| Thermochemistry | |||
Heat capacity (C) |
1.468 J·g−1·K−1 | ||
| Hazards | |||
| Main hazards | Corrosive - causes burns | ||
| Safety data sheet | MSDS | ||
| Flash point | 64 °C (147 °F; 337 K) | ||
| 550 °C (1,022 °F; 823 K) | |||
| Related compounds | |||
Related aromatic hydrocarbons |
benzene phenol chlorobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
See also
References
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 3–120, ISBN 0-8493-0594-2
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, p. 1281, ISBN 0-8493-0594-2
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 8–103, ISBN 0-8493-0594-2
- Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.), Boca Raton, Florida: CRC Press, pp. 15–18, ISBN 0-8493-0594-2
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
- Fiege, H.; Voges, H.-M.; Hamamoto, T; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D. (2000). "Phenol Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_313.
External links
- ToxFAQs for Chlorophenols, Agency for Toxic Substances and Disease Registry.
- Compound Summary Compendium, PubChem Open Chemistry Database.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.