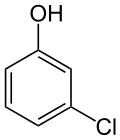
3-Chlorophenol
3-Chlorophenol is an organic compound with the molecular formula ClC6H4OH. It is one of three isomers of monochlorophenol. It is a colorless or white solid that melts easily and exhibits significant solubility in water. Together with 3,5-dichlorophenol, it is prepared industrially by dechlorination of polychlorophenols. Alternatively, it arises via the cumene process, which starts with the alkylation of chlorobenzene with propylene.[2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3-Chlorophenol[1] | |
| Other names
m-Chlorophenol meta-Chlorophenol 3-Hydroxychlorobenzene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.003.257 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H5ClO | |
| Molar mass | 128.56 g·mol−1 |
| Appearance | Colorless or white oily solid |
| Density | 1.245 g/cm3 at 20 °C |
| Melting point | 30 to 34 °C (86 to 93 °F; 303 to 307 K) |
| Boiling point | 214 °C (417 °F; 487 K) |
| 20 g/L at 20 °C | |
| Solubility in other solvents | Soluble in ethanol, diethyl ether, benzene |
| Vapor pressure | kPa |
| Acidity (pKa) | 8.97 |
| Hazards | |
| Main hazards | Corrosive - causes burns |
| Safety data sheet | MSDS |
| Flash point | 120 °C (248 °F; 393 K) |
| 550 °C (1,022 °F; 823 K) | |
| Related compounds | |
Related aromatic hydrocarbons |
Benzene Phenol Chlorobenzene |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 690. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
Only one name is retained, phenol, for C6H5-OH, both as a preferred name and for general nomenclature. The structure is substitutable at any position. Locants 2, 3, and 4 are recommended, not o, m, and p.
- François Muller; Liliane Caillard (2011). "Chlorophenols". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_001.pub2.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.