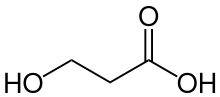
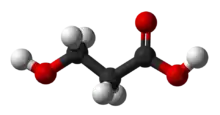
3-Hydroxypropionic acid
3-Hydroxypropionic acid is a carboxylic acid, specifically a beta hydroxy acid. It is an acidic viscous liquid with a pKa of 4.5.[1] It is very soluble in water, soluble in ethanol and diethyl ether. Upon distillation, it dehydrates to form acrylic acid, and is occasionally called hydracrylic acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
3-Hydroxypropanoic acid | |
| Other names
3-hydroxypropionic acid hydracrylic acid ethylene lactic acid | |
| Identifiers | |
3D model (JSmol) |
|
| 3DMet | |
| 773806 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.250 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H6O3 | |
| Molar mass | 90.08 g/mol |
| Melting point | <25 °C 143 °C (sodium salt) |
| Boiling point | Decomposes |
| Very soluble | |
| Acidity (pKa) | 4.87[2] |
| Related compounds | |
Related carboxylic acids |
acetic acid glycolic acid propionic acid lactic acid malonic acid butyric acid hydroxybutyric acid |
Related compounds |
1-propanol 2-propanol propionaldehyde acrolein |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
3-Hydroxypropionic acid is used in the industrial production of various chemicals such as acrylates. It can be produced by engineered microbes.[3]
Applications in producing a biodegradable polymer
A method has been developed by the University of Minnesota to produce a biodegradable polymer polyester known as poly(3-hydroxypropionic acid).[4] The method combines the high-molecular weight and control aspects of ring-opening polymerization with the commercial availability of the beta hydroxy acid, 3-hydroxypropionic acid which is abbreviated as 3-HP. Since 3-HP can be derived from biological sources, the resulting material, poly(3-hydroxypropionic acid) or P(3-HP), is biorenewable. The new method allows direct synthesis of the bio-based polymer P(3-HP) from 3-HP, a commercial monomer that is derived from corn. The method uses a single vessel reactor for simple synthesis and rapid scale up. The method results in a higher molecular weight which makes the polymer more structurally sound using a process with lower toxicity than competing technologies.
The market for a bio-based and biodegradable replacement for polyester is expected to grow rapidly during the next five years. The bio-based polyester, P(3-HP), has attractive mechanical properties, such as rigidity, ductility, and exceptional tensile strength in drawn films and can be created using the new lower toxicity method. On account of these properties, P(3-HP) has applications in packaging or biodegradable plastics.
Genetically encoded 3-hydroxypropionic acid inducible system
A genetically encoded 3-hydroxypropionic acid inducible system has been characterized in bacteria demonstrating that such system in combination with fluorescent reporter protein can be utilized as a biosensor to measure intracellular and extracellular 3-HP concentrations by fluorescence output.[5]
See also
- Lactic acid (2-hydroxypropanoic acid)
- listed as hydracrylic acid in the Merck index, 12th Edition
References
- Merck Index, 11th Edition, 4681.
- Handbook of Chemistry and Physics, CRC press, 58th edition page D150-151 (1977)
- "3-HP". Retrieved 27 May 2011.
- Hanko, E.K.R.; Minton, N.P.; Malys, N. (2017). "Characterisation of a 3-hydroxypropionic acid-inducible system from Pseudomonas putida for orthogonal gene expression control in Escherichia coli and Cupriavidus necator". Scientific Reports. 7 (1724). doi:10.1038/s41598-017-01850-w. PMC 5431877.
External links
- Chisholm, Hugh, ed. (1911). . Encyclopædia Britannica. 14 (11th ed.). Cambridge University Press. p. 34.