4,4'-Methylenedianiline
4,4'-Methylenedianiline (MDA) is an organic compound with the formula CH2(C6H4NH2)2. It is a colorless or white solid. It is produced on industrial scale as a precursor to polyurethanes.
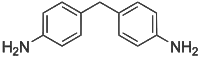 | |
| Names | |
|---|---|
| IUPAC name
Bis(4-aminophenyl)methane | |
| Other names
4,4'-Diaminodiphenylmethane; 4,4'-Methylenebisbenzenamine; MDA; para, para'-Diaminodiphenyl-methane; Dianilinomethane; 4,4'-Diphenylmethanediamine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.705 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2651 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C13H14N2 | |
| Molar mass | 198.269 g·mol−1 |
| Appearance | Pale brown, crystalline solid |
| Odor | faint, amine-like[1] |
| Density | 1.05 g/cm3 (100°C) |
| Melting point | 89 °C (192 °F; 362 K) |
| Boiling point | 398 to 399 °C (748 to 750 °F; 671 to 672 K) |
| 0.125 g/100 ml (20 °C) | |
| Vapor pressure | 0.0000002 mmHg (20°C)[1] |
| Hazards | |
| Main hazards | potential carcinogen[1] |
| Flash point | 190 °C; 374 °F; 463 K [1] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
TWA 0.010 ppm ST 0.100 ppm[1] |
REL (Recommended) |
Ca[1] |
IDLH (Immediate danger) |
Ca [N.D.][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Synthesis and applications
In the industrial production, MDA is produced by reaction of formaldehyde and aniline in the presence of hydrochloric acid.[2]
MDA is consumed mainly as a precursor to 4,4 ́-methylene diphenyl diisocyanate (MDI). MDA is treated with phosgene to give MDI. MDI is a precursor to many polyurethane foams.[3][4] Lower quantities are used as hardeners in epoxy resins and adhesives, as well as in the production of high-performance polymers.[2] MDA is hydrogenated to give 4,4-diaminodicyclohexylmethane, which is also used in polymer chemistry.[5]
Safety
MDA is considered a potential occupational carcinogen by the US National Institute for Occupational Safety and Health. The Occupational Safety and Health Administration has set a permissible exposure limit at 0.01 ppm over an eight-hour time-weighted average, and a short-term exposure limit at 0.10 ppm.[6]
It is suspected carcinogen.[3] It is included in the "substances of very high concern" list of the European Chemicals Agency (ECHA).[4] The compound was blamed in a mass poisoning in the vicinity of Epping, Essex, United Kingdom during 1965 during which 84 individuals were poisoned through accidental contamination of flour used to make bread.[7]
References
- NIOSH Pocket Guide to Chemical Hazards. "#0415". National Institute for Occupational Safety and Health (NIOSH).
- "Data on manufacture, import, export, uses and release of 4-4' diaminodiphenylmethane" (PDF). Archived from the original (PDF) on 2011-10-01.
- "ToxFAQs for 4,4'-Methylenedianiline". Agency for Toxic Substances and Disease Registry.
- "Background document for 4,4'-Diaminodiphenylmethane (MDA)" (PDF). European Chemicals Agency.
- Roose P, Eller K, Henkes E, Rossbacher R, Höke H (2005). "Amines, Aliphatic". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001.
- "4,4'-Methylenedianiline". NIOSH Pocket Guide on Chemical Hazards.
- Kopelman H, Robertson MH, Sanders PG, Ash I (February 1966). "The Epping jaundice". British Medical Journal. 1 (5486): 514–6. doi:10.1136/bmj.1.5486.514. PMC 1843808. PMID 5902696.
External links
- "International Labour Organization". icsc1111.
- "NIOSH Pocket Guide to Chemical Hazards". Centers for Disease Control and Prevention.
- "European Union Risk Assessment Report" (PDF).
- Petersen JH, Mortensen SK, Pedersen GA (12 October 2004). "An acute case of primary aromatic amines migrating from cooking utensils" (PDF). Memorandum for the Danish Veterinary and Food Administration on. Danish Institute for Food and Veterinary Research.