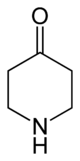
4-Piperidinone
4-Piperidinone is a derivative of piperidine with the molecular formula C5H9NO. 4-Piperidone is used as an intermediate in the manufacture of chemicals and pharmaceutical drugs.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Piperidin-4-one | |
| Other names
4-Piperidone Azinanone Azinan-4-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.050.420 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H9NO | |
| Molar mass | 99.133 g·mol−1 |
| Boiling point | 79 °C (174 °F; 352 K) |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 91 °C (196 °F; 364 K) |
| Related compounds | |
Related compounds |
Piperidine; 2-Piperidinone |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Piperidones
Piperidones are a class of chemical compounds sharing the piperidone skeleton. A classic named reaction for the synthesis of piperidones is the Petrenko-Kritschenko piperidone synthesis which involves combining an alkyl-1,3-acetonedicarboxylate with benzaldehyde and an amine.[1] This multicomponent reaction is related to the Hantzsch pyridine synthesis.
See also
References
- Petrenko-Kritschenko P, Zoneff N (March 1906). "Ueber die Condensation von Aceton‐dicarbonsäureestern mit Benzaldehyd unter Anwendung von Ammoniak". Berichte der Deutschen Chemischen Gesellschaft (in German). 39 (2): 1358–61. doi:10.1002/cber.19060390234.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.
