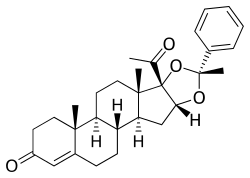
Algestone acetophenide
Algestone acetophenide, also known more commonly as dihydroxyprogesterone acetophenide (DHPA) and sold under the brand names Perlutal and Topasel among others, is a progestin medication which is used in combination with an estrogen as a form of long-lasting injectable birth control.[3][4][5][6] It has also been used alone, but is no longer available as a standalone medication.[7][8][9] DHPA is not active by mouth and is given once a month by injection into muscle.[4][5][6]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Perlutal, Topasel, Unalmes, Yectames, many others |
| Other names | Dihydroxyprogesterone acetophenide; DHPA; Deladroxone; Droxone; Alfasone acetophenide; Alphasone acetophenide; SQ-15101; 16α,17α-Dihydroxyprogesterone acetophenide; 16α,17α-Dihydroxypregn-4-ene-3,20-dione cyclic acetal with acetophenone; (R)-16α,17-[(1-Phenylethylidene)dioxy]pregn-4-ene-3,20-dione |
| Routes of administration | Intramuscular injection |
| Drug class | Progestogen; Progestin |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | IM: 24 days[1][2] |
| Excretion | Preferentially feces[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.981 |
| Chemical and physical data | |
| Formula | C29H36O4 |
| Molar mass | 448.603 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Side effects of DHPA are similar to those of other progestins. DHPA is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[10][11][12] It has no other important hormonal activity.[10][13][11][12]
DHPA was discovered in 1958 and was introduced for medical use in the 1960s.[14][15][16] It was not introduced in the United States, but it is marketed widely throughout Latin America.[17][18][7][16] It was also previously available alone in Italy and as a combined injectable contraceptive in Portugal and Spain, but has been discontinued in these countries.[8]
Medical uses
DHPA is used in combination with estradiol enantate (E2-EN) or estradiol benzoate butyrate (EBB) as a once-monthly combined injectable contraceptive for women in Latin America, Hong Kong, and Singapore.[4][5][6] It was also previously marketed for use alone in Italy.[7] DHPA has reportedly been used to treat acne.[19][20] E2-EN/DHPA has been said to be used by "travestis" (a term for transgender women in some cultures, especially in South America) as a means of feminizing hormone therapy as well.[21]
Available forms
The following forms of DHPA in combination with an estrogen are or have been available for use:[5][22][23][24][25][4]
- 150 mg DHPA and 10 mg estradiol enantate (brand names Perlutal, Topasel, many others)
- 75 mg DHPA and 5 mg estradiol enantate (brand names Anafertin, Patector NF, Yectames)
- 120 mg DHPA and 10 mg estradiol enantate (brand names Unalmes, Yectuna)
- 75 mg DHPA and 10 mg estradiol enantate (brand name Ova Repos; discontinued)
- 150 mg DHPA and 10 mg estradiol benzoate butyrate (brand names Neolutin N, Redimen, Soluna, Unijab)
A 90 mg DHPA and 6 mg estradiol enantate formulation was also studied, but was never marketed.[26][27][28] The combination of DHPA and estradiol enantate has also been studied at other doses ranging from 75 to 200 mg DHPA and 5 to 50 mg estradiol enantate.[29]
Side effects
Side effects of DHPA are similar to those of other progestins. Side effects of the combination of DHPA and estradiol enantate have reportedly included dysmenorrhea, breast tenderness, headache, edema, bloating, changes in libido, depression, anxiety, and injection site pain.[29][4] The half-dose formulation of DHPA and estradiol enantate retains contraceptive effects but causes severe disruption of menstrual bleeding patterns.[1][30] Likewise, the formulation of DHPA in combination with estradiol benzoate butyrate has been associated with poor control of menstrual bleeding.[25][4]
Overdose
DHPA has been studied at high doses of 900 mg/week by intramuscular injection in women with endometrial cancer.[31]
Pharmacology
Pharmacodynamics
DHPA is a progestogen, or an agonist of the progesterone receptor.[10][11][12] It is said to be both more potent and longer-acting than the related progestogen hydroxyprogesterone caproate.[32] The progestogenic potency of DHPA is about 2 to 5 times that of progesterone in animals.[3] The medication has no androgenic, antiandrogenic, estrogenic, antiestrogenic, glucocorticoid, or antimineralocorticoid activities, and hence is a pure progestogen with no off-target activity.[1][4][13][10][11][12]
Clinical studies have found that, on the basis of endometrial changes, E2-EN/DHPA appears, at the doses used, to be an estrogen-dominant combination.[13]
| Compound | Form | Dose for specific uses (mg)[lower-alpha 3] | DOA[lower-alpha 4] | |||
|---|---|---|---|---|---|---|
| TFD[lower-alpha 5] | POICD[lower-alpha 6] | CICD[lower-alpha 7] | ||||
| Algestone acetophenide | Oil soln. | - | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[lower-alpha 8] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[lower-alpha 9] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | - | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[lower-alpha 9] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||
Pharmacokinetics
The pharmacokinetics of DHPA have been studied, albeit limitedly.[1][50][51] One study in women observed an elimination half-life of DHPA and its metabolites of 24 days and found that it remained detectable in the circulation for up to 60 days following a single intramuscular injection.[51][1][2] In another study, the duration of action of DHPA was reported to be more than 100 days after a single subcutaneous injection, although it was unspecified as to whether this was in humans or animals.[1] DHPA is excreted preferentially in feces via the biliary route.[13][2][3]
Chemistry
DHPA, also known as 16α,17α-dihydroxyprogesterone acetophenide, as well as 16α,17α-dihydroxypregn-4-ene-3,20-dione cyclic acetal with acetophenone or as (R)-16α,17-[(1-phenylethylidene)dioxy]pregn-4-ene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone.[52][7][53] It is specifically a derivative of 17α-hydroxyprogesterone with an additional hydroxyl group at the C17α position, or of algestone (16α,17α-dihydroxyprogesterone), and with the two hydroxyl groups cyclized into an acetophenide moiety (a cyclic acetal with acetophenone).[52][7][53] Analogues of DHPA include other 17α-hydroxyprogesterone derivatives such as algestone acetonide (dihydroxyprogesterone acetonide), chlormadinone acetate, cyproterone acetate, gestonorone caproate, hydroxyprogesterone caproate, medroxyprogesterone acetate, megestrol acetate, and segesterone acetate.[52][7]
Synthesis
Chemical syntheses of DHPA have been published.[54][53][55]
History
DHPA was first described in the literature in 1958 and was patented in 1960.[14][15] It was developed in combination with estradiol enantate as a long-lasting combined injectable contraceptive under the tentative brand names Deladroxate and Droxone by Squibb and was studied in women starting in 1964.[56][57][58][26] Development was discontinued by Squibb in the United States in the late 1960s due to concerns of toxicological findings in animals, including mammary gland tumors in beagle dogs and pituitary hyperplasia in rats, as well as possible accumulation of estradiol enantate in the body with continued use.[1][17][16] Subsequent research has shed doubt that these animal findings are applicable to humans and that the dosages required for contraception would pose any risks.[17][1] Although the medication was not marketed in the United States, its development was continued elsewhere and it went on to be introduced and widely used in Latin America and Spain.[18][7][16] A standalone version of DHPA was introduced in Italy in 1982 under the brand names Neolutin Depo and Neolutin Depositum.[53][7] This single-drug formulation has since been discontinued.[8][9] DHPA remains available in Latin America, but is no longer marketed in Europe.[8][9]
Society and culture
Generic names
Algestone acetophenide are the English generic name of the drug and its INNM and USAN, while dihydroxyprogesterone acetophenide (DHPA) is a commonly used synonym.[52][59][60][8][9][61] Its generic names in other languages are as follows:[52][59][60][8][9][61]
- French: acétophénide d'algestone and dihydroxyprogestérone acétophénide
- German: algeston acetofenid and dihydroxyprogesteron acetophenid
- Italian: algestone acetofenide and diidrossiprogesterone acetofenide
- Portuguese and Spanish: acetofenido de algestona, algestona acetofenido, acetofenido de dihidroxiprogesterona, and dihidroxiprogesterona acetofenido
DHPA is also known by its former developmental code name SQ-15101.[52][7][59][60] It has been referred to as deladroxone, droxone, alfasone acetophenide, and alphasone acetophenide as well.[52][54][53][62][63][59][60][7]
Brand names
DHPA has been marketed alone and in combination with estrogens under a wide variety of brand names.[8][9][59][60][7][53][5][18][23][16][25][4][50] It was marketed alone under the brand name Neolutin Depositum, but this preparation was discontinued.[8][7][54][53] The medication was developed under the developmental code names Deladroxone and Droxone, but these brand names were never used commercially.[52][62][63] DHPA has been marketed in combination with estradiol enantate (E2-EN) as a combined injectable contraceptive in a few different preparations, with varying doses of E2-EN and DHPA.[23][5][18][22][25][4][50] These formulations all have different brand names, which include the following († = discontinued):[8][9][59][60][22][23][5][18][4][64]
- E2-EN 10 mg / DHPA 150 mg: Acefil, Agurin†, Atrimon†, Ciclomes, Ciclovar, Ciclovular, Cicnor†, Clinomin, Cycloven, Daiva, Damix, Deprans, Deproxone, Exuna, Ginestest, Ginoplan†, Gynomes, Horprotal, Listen, Luvonal, Neogestar, Neolutin, Nomagest, Nonestrol, Normagest, Normensil, Novular, Oterol, Ovoginal, Patector, Patectro, Perludil, Perlumes, Perlutal, Perlutale, Perlutan, Perlutin, Perlutin-Unifarma, Permisil, Preg-Less, Pregnolan, Progestrol†, Protegin, Proter, Seguralmes, Synovular, Topasel, Unigalen, Uno-Ciclo, and Vagital.
- E2-EN 10 mg / DHPA 120 mg: Anafertin†, Patector NF, and Yectames.
- E2-EN 5 mg / DHPA 75 mg: Unalmes and Yectuna.
- E2-EN 10 mg / DHPA 75 mg: Ova Repos†.
- Unsorted: Evitas†, Femineo†, and Primyfar†.
The combination of E2-EN 10 mg and DHPA 150 mg was developed under the developmental brand name Deladroxate, but this brand name was never used commercially.[25][4]
In addition to E2-EN, DHPA is marketed in combination with estradiol benzoate butyrate (EBB) as a combined injectable contraceptive under the brand names Neolutin N, Redimen, Soluna, and Unijab.[22][23][24] This combination was developed under the developmental brand name Unimens, but this brand name was never used commercially.[25][65]
DHPA has also been used in veterinary medicine in cows under the brand name Bovitrol.[52][62][66][67][68]
Availability
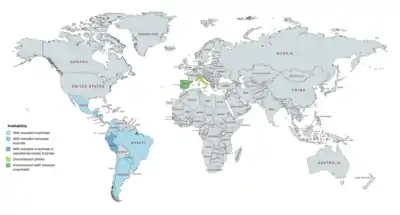
DHPA has been available for use both alone and in combination with estrogens.[8][9][25][59][60][7] It was marketed alone under the brand name Neolutin Depositum in Italy, but this preparation was discontinued.[8][7][53] DHPA has been marketed in combination with estradiol enantate (E2-EN) as a combined injectable contraceptive in at least 19 countries, mostly in Latin America.[5][18][23][16][8][9][59][60] A few different preparations, with varying doses of E2-EN and DHPA and varying availability, have been introduced.[23][5][18][22][25][4][50] These formulations have the following approval and availability († = discontinued in this country):[8][9][59][60][22][23][5][18][4]
- E2-EN 10 mg / DHPA 150 mg: at least 19 countries, including Argentina, Belize, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Hong Kong, Mexico, Nicaragua, Panama, Paraguay, Peru, Portugal†, and Spain†.
- E2-EN 10 mg / DHPA 120 mg: at least 3 countries, including Brazil†, Chile, and Paraguay.
- E2-EN 5 mg / DHPA 75 mg: at least 9 countries, including Costa Rica, the Dominican Republic, El Salvador, Guatemala, Honduras, Mexico, Nicaragua, Panama, and Spain†.
In addition to E2-EN, DHPA is marketed in combination with estradiol benzoate butyrate (EBB) as a combined injectable contraceptive in Peru and Singapore.[22][23][24] EBB has a shorter duration than E2-EN of about 3 weeks and hence EBB/DHPA was developed because it was thought that it would be more suitable for use as a once-monthly combined injectable contraceptive than E2-EN/DHPA.[65]
Usage
E2-EN/DHPA is the most widely used combined injectable contraceptive in Latin America.[69] It was estimated in 1995 that E2-EN/DHPA was used as a combined injectable contraceptive in Latin America by at least 1 million women.[23] However, combined injectable contraceptives like E2-EN/DHPA are unlikely to constitute a large proportion of contraceptive use in the countries in which they are available.[23]
Research
DHPA was studied by its developer Squibb for use as a progestogen-only injectable contraceptive at a dose of 100 mg once per month by intramuscular injection under the developmental code name and tentative brand name Deladroxone.[65][13] It was associated with poor cycle control and was never marketed for this indication.[13]
See also
References
- Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- "Bula do Algestona Acetofenida + Enantato de Estradiol". Consulta Remédios. Archived from the original on 2018-09-18. Retrieved 2018-09-18.
- Jarquín González JD, Elda de Aguirre L, Rodríguez C, Abrego de Aguilar M, Carrillo F, León DA, Lima M, Trigueros S, Acosta R (September 1996). "Dihydroxyprogesterone acetophenide 150 mg + estradiol enantate 10 mg as monthly injectable contraceptives". Adv Contracept. 12 (3): 213–25. doi:10.1007/BF01849664. PMID 8910663. S2CID 2522426.
- Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 Suppl 1: S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357. Archived from the original (PDF) on 2017-08-10. Retrieved 2016-08-24.
- Rowlands, S (2009). "New technologies in contraception" (PDF). BJOG: An International Journal of Obstetrics & Gynaecology. 116 (2): 230–239. doi:10.1111/j.1471-0528.2008.01985.x. ISSN 1470-0328. PMID 19076955. S2CID 3415547.
- Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 26–. ISBN 978-3-88763-075-1.
- http://www.micromedexsolutions.com/micromedex2/librarian/
- Sweetman, Sean C., ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- Lerner LJ, Brennan DM, Borman A (1961). "Biological activities of 16 alpha, 17 alpha dihydroxyprogesterone derivatives". Proc. Soc. Exp. Biol. Med. 106: 231–4. doi:10.3181/00379727-106-26296. PMID 13761080. S2CID 89428532.
- Lerner, L. J., Brennan, D. M., DePhillipo, M., & Yiacas, E. (1961). Comparison of biological activities of progesterone, norethisterone and the acetophenone derivatives of 16 alpha, 17 alpha-dihydroxyprogesterone.(Abstr.). In Federation Proceedings (Vol. 20, p. 200).
- Kurihara N, Tadokoro S, Ogawa H (1972). "Studies on hormonal actions of dihydroxyprogesterone acetophenide, estradiol enanthate and their mixtures". Jpn. J. Pharmacol. 22 (1): 43–58. doi:10.1254/jjp.22.43. PMID 4537624.
- Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- Fried, J. (1960). U.S. Patent No. 2,941,997. Washington, DC: U.S. Patent and Trademark Office.
- Fried J, Borman A (1958). "Synthetic derivatives of cortical hormones". Vitam. Horm. Vitamins & Hormones. 16: 303–74. doi:10.1016/S0083-6729(08)60320-9. ISBN 9780127098166. PMID 13625604.
- Thomas Rabe; Benno Runnebaum (6 December 2012). Fertility Control — Update and Trends: Update and Trends. Springer Science & Business Media. pp. 183–. ISBN 978-3-642-86696-8.
Two additional monthly, combined injectable methods warrant mention. Deladroxate (commercially labelled as Perlutan, Topasel, Agurin, Horprotal and Uno-Ciclo in various countries), is a combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate, and is available in many Latin American countries and Spain. The method is highly effective, without a single pregnancy reported in large clinical trials (Koetsawang 1994). Although available since the 1960s, the method has not been studied as extensively as Cyclofem or Mesigyna. The original manufacturer withdrew support due to toxicological concerns with dihydroxyprogesterone acetophenide, and clinical evaluations continue to be published. A recent dose-finding trial compared the standard available dose of 150/10 with a lower dose of 90/6, and concluded the lower dose was equally effective (Coutinho et al., 1997).
- Population Reports: Injectables and implants. Department of Medical and Public Affairs, George Washington University. 1987. p. K-75.
In the US, Squibb Pharmaceutical Company withdrew Deladroxate from clinical testing in the late 1960s because of concerns over (1) breast tumors in beagle dogs, (2) pituitary hyperplasia in rats, and (3) possible accumulation of estradiol enanthate in the body with continued use (89, 98, 243). Subsequently, however, questions have been raised about whether such animal findings are applicable to humans. Research suggests that the adverse effects of Deladroxate on animals may occur only with doses higher than the equivalent of a contraceptive dose (2, 62, 82, 121, 272).
- Pramilla Senanayake; Malcolm Potts (14 April 2008). Atlas of Contraception, Second Edition. CRC Press. pp. 50–. ISBN 978-0-203-34732-4.
- George W.A Milne (8 May 2018). Drugs: Synonyms and Properties: Synonyms and Properties. Taylor & Francis. pp. 1565–. ISBN 978-1-351-78989-9.
- Pei-Show Juo (21 December 2001). Concise Dictionary of Biomedicine and Molecular Biology. CRC Press. pp. 57–. ISBN 978-1-4200-4130-9.
- Don Kulick (12 January 2009). Travesti: Sex, Gender, and Culture among Brazilian Transgendered Prostitutes. University of Chicago Press. pp. 64–66. ISBN 978-0-226-46101-4.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 431–433, 467. ISBN 978-92-832-1291-1.
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- http://www.mims.com/singapore/drug/info/unijab
- Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- d’Arcangues, Catherine; Snow, Rachel C. (1999). "Injectable Contraceptives". Fertility Control — Update and Trends. pp. 121–149. doi:10.1007/978-3-642-86696-8_6. ISBN 978-3-642-86698-2.
- Coutinho EM, Spinola P, Barbosa I, Gatto M, Tomaz G, Morais K, Yazlle ME, de Souza RN, Pinho Neto JS, Leal Wde B, Leal C, Hippolito SB, Abranches AD (March 1997). "Multicenter, double-blind, comparative clinical study on the efficacy and acceptability of a monthly injectable contraceptive combination of 150 mg dihydroxyprogesterone acetophenide and 10 mg estradiol enanthate compared to a monthly injectable contraceptive combination of 90 mg dihydroxyprogesterone acetophenide and 6 mg estradiol enanthate". Contraception. 55 (3): 175–81. doi:10.1016/S0010-7824(97)00018-8. PMID 9115007.
- Coutinho EM, Spinola P, Tomaz G, Morais K, Nassar de Souza R, Sabino Pinho Neto J, de Barros Leal W, Bomfim Hippolito S, D'Aurea Abranches A (April 2000). "Efficacy, acceptability, and clinical effects of a low-dose injectable contraceptive combination of dihydroxyprogesterone acetophenide and estradiol enanthate". Contraception. 61 (4): 277–80. doi:10.1016/S0010-7824(00)00099-8. PMID 10899484.
- Koetsawang S (April 1994). "Once-a-month injectable contraceptives: efficacy and reasons for discontinuation". Contraception. 49 (4): 387–98. doi:10.1016/0010-7824(94)90034-5. PMID 8013221.
- Recio R, Garza-Flores J, Schiavon R, Reyes A, Diaz-Sanchez V, Valles V, Luz de la Cruz D, Oropeza G, Perez-Palacios G (June 1986). "Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive". Contraception. 33 (6): 579–89. doi:10.1016/0010-7824(86)90046-6. PMID 3769482.
- Kennedy BJ (July 1968). "Progestogens in the treatment of carcinoma of the endometrium". Surg Gynecol Obstet. 127 (1): 103–14. PMID 5657772.
- Kawakami M, Sawyer CH (1967). "Effects of sex hormones and antifertility steroids in brain thresholds in the rabbit". Endocrinology. 80 (5): 857–71. doi:10.1210/endo-80-5-857. PMID 4164655.
- Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- A. Labhart (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- Joachim Ufer (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- Willibald Pschyrembel (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- Janet Brotherton (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–5. doi:10.1159/000280353. PMID 6452729.
- Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe Und Frauenheilkunde. 43 (5): 281–7. doi:10.1055/s-2008-1036893. PMID 6223851.
- Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- Gual, C.; Pérez-Palacios, G.; Pérez, A.E.; Ruiz, M.R.; Solis, J.; Cervantes, A.; Iramain, C.; Schreiber, E.C. (1973). "Metabolic fate of a long-acting injectable estrogen-progestogen contraceptive 1,2". Contraception. 7 (4): 271–287. doi:10.1016/0010-7824(73)90145-5. ISSN 0010-7824.
- J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 27–. ISBN 978-1-4757-2085-3.
- William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 153–. ISBN 978-0-8155-1856-3.
- Axel Kleemann; Jürgen Engel (2001). Pharmaceutical Substances: Syntheses, Patents, Applications. Thieme. p. 61. ISBN 978-3-13-558404-1.
- Die Gestagene. Springer-Verlag. 27 November 2013. pp. 8–9. ISBN 978-3-642-99941-3.
- Rutherford RN, Banks AL, Coburn WA (1964). "Deladroxate for the Prevention of Ovulation". Fertil. Steril. 15 (6): 648–52. doi:10.1016/s0015-0282(16)35410-3. PMID 14236841.
- Taymor ML, Planck S, Yahia C (1964). "Ovulation Inhibition with a Long-Acting Parenteral Progestogen-Estrogen Combination". Fertil. Steril. 15 (6): 653–60. doi:10.1016/s0015-0282(16)35411-5. PMID 14236842.
- Paolo Giovanni Artini; Andrea R. Genazzani; Felice Petraglia (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 101–. ISBN 978-1-84214-071-0.
Subseuqently another formulation of 150 mg of dihydroxyprogesterone acetophenide (DHPA) with 10 mg estradiol enanthate (E2-EN) was tested in the 1960s3-6.
- https://www.drugs.com/international/algestone.html
- https://www.drugs.com/international/algestone-acetophenide.html
- I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 10–. ISBN 978-94-011-4439-1.
- Martin Negwer (1994). Organic-chemical Drugs and Their Synonyms: (an International Survey). Akademie Verlag. p. 2572. ISBN 978-3-05-500156-7.
Algestone acetophenide, Alphasone acetophenide, Bovitrol, Deladroxone, Dihydroxyprogesterone acetophenide, Droxone, Neolutin depositum, SQ 15101 U Progestin, spermatogenesis inhibitor
- Chemical Contraception. Macmillan International Higher Education. 18 June 1974. pp. 55–. ISBN 978-1-349-02287-8.
- Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". Cochrane Database Syst Rev. 3: CD004568. doi:10.1002/14651858.CD004568.pub3. PMC 6513542. PMID 23641480.
- Mokhtar K. Toppozada (1983). "Monthly Injectable Contraceptives". In Alfredo Goldsmith; Mokhtar Toppozada (eds.). Long-Acting Contraception. pp. 93–103. OCLC 35018604.
- North Dakota State University. Extension Service (1967). Circulars. p. XV.
- Louisiana Agriculture. Agricultural Experiment Station, Louisiana State University and Agricultural and Mechanical College. 1967. p. 38.
- Beef Cattle Science Handbook. Agriservices Foundation. 1971. p. 148.
- Leon Speroff; Marc A. Fritz (2005). Clinical Gynecologic Endocrinology and Infertility. Lippincott Williams & Wilkins. pp. 969–. ISBN 978-0-7817-4795-0.