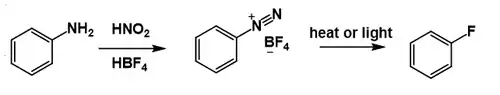
Balz–Schiemann reaction
The Schiemann reaction (also called the Balz–Schiemann reaction) is a chemical reaction in which a primary aromatic amine is transformed to an aryl fluoride via a diazonium tetrafluoroborate intermediate.[1] This reaction is a traditional route to fluorobenzene and some related derivatives,[2] including 4-fluorobenzoic acid.[3]
| Balz-Schiemann reaction | |
|---|---|
| Named after | Günther Balz Günther Schiemann |
| Reaction type | Substitution reaction |
| Identifiers | |
| Organic Chemistry Portal | balz-schiemann-reaction |
| RSC ontology ID | RXNO:0000127 |
The reaction is conceptually similar to the Sandmeyer reaction, which converts diazonium salts to other aryl halides (ArCl, ArBr).[4] However, while the Sandmeyer reaction involves a copper reagent/catalyst and radical intermediates, the thermal decomposition of the diazonium tetrafluoroborate proceeds without a promoter and is believed to generate highly unstable aryl cations (Ar+), which abstract F− from BF4− to give the fluoroarene (ArF), along with boron trifluoride as the byproduct.
Innovations
The traditional Balz–Schiemann reaction employs HBF4 and involves isolation of the diazonium salt. Both aspects can be profitably modified. Other counterions have been used in place of tetrafluoroborates, such as hexafluorophosphates (PF6−) and hexafluoroantimonates (SbF6−) with improved yields for some substrates. The diazotization reaction can be effected with nitrosonium salts such as [NO]SbF6 without isolation of the diazonium intermediate.[1]
History
The reaction is named after the German chemists Günther Schiemann and Günther Balz.[5]
Additional literature
- Roe A (1949). "Preparation of Aromatic Fluorine Compounds from Diazonium Fluoborates". Org. React. 5: 193. doi:10.1002/0471264180.or005.04. ISBN 0471264180.
- Becker H. G. O., Israel G. (1978). "Ionenpaareffekte bei der Photolyse und Thermolyse von Aryldiazonium-tetrafluoroboraten". J. Prakt. Chem. 321 (4): 579–586. doi:10.1002/prac.19793210410.
References
- Furuya, Takeru; Klein, Johannes E. M. N.; Ritter, Tobias (2010). "C–F Bond Formation for the Synthesis of Aryl Fluorides". Synthesis. 2010 (11): 1804–1821. doi:10.1055/s-0029-1218742. PMC 2953275. PMID 20953341.
- Flood, D. T. (1943). "Fluorobenzene". Organic Syntheses.; Collective Volume, 2, p. 295
- G. Schiemann; W. Winkelmüller (1943). "p-Fluorobenzoic Acid". Organic Syntheses.; Collective Volume, 2, p. 299
- Swain, C. G.; Rogers, R. J. (1975). "Mechanism of formation of aryl fluorides from arenediazonium fluoborates". J. Am. Chem. Soc. 97 (4): 799–800. doi:10.1021/ja00837a019.
- Günther Balz; Günther Schiemann (1927). "Über aromatische Fluorverbindungen, I.: Ein neues Verfahren zu ihrer Darstellung". Chemische Berichte. 5 (60): 1186–1190. doi:10.1002/cber.19270600539.