Borate
Borates are boron-oxygen compounds, which form boron oxyanions. These can be trigonal or tetrahedral in structure, or more loosely can consist of chemical mixtures which contain borate anions of either description. The element boron most often occurs in nature as borates, such as borate minerals and borosilicates.
Structures
Borates are composed of trigonal planar BO3 or tetrahedral BO4 structural units, joined together via shared oxygen atoms[1] and may be cyclic or linear in structure.
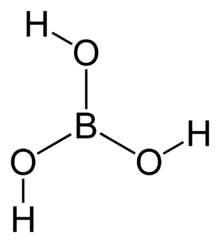
The simplest borate anion, the orthoborate(3−) ion, [BO3]3−, is known in the solid state, for example, in Ca3(BO3)2,[2] where it adopts a nearly trigonal planar structure. It is a structural analogue of the carbonate anion [CO3]2−, with which it is isoelectronic. Simple bonding theories point to the trigonal planar structure. In terms of valence bond theory, the bonds are formed by using sp2 hybrid orbitals on boron. Some compounds termed orthoborates do not necessarily contain the trigonal planar ion, for example, gadolinium orthoborate GdBO3 contains the polyborate [B3O9]9− ion, whereas the high-temperature form contains planar [BO3]3−.[3]
Boric acid

All borates can be considered derivatives of boric acid, B(OH)3. Boric acid is a weak proton donor (pKa ~ 9) in the sense of Brønsted acid, but is a Lewis acid, i.e., it can accept an electron pair. In water, it behaves as a Lewis acid, accepting the electron pair of a hydroxyl ion produced by the water autoprotolysis.
B(OH)3 is acidic because of its reaction with OH− from water, forming the tetrahydroxyborate complex [B(OH)4]− and releasing the corresponding proton left by the water autoprotolysis:[4]
- B(OH)3 + 2 H2O ⇌ [B(OH)4]− + [H3O]+ (pKa = 8.98)[5]
In the presence of cis-vicinal diols, such as mannitol, sorbitol, glucose and glycerol, the acidity of the boric acid solution is increased, and the pKa can be lowered to about 4 if enough mannitol is added.[6]
With different mannitol concentrations, the pK of B(OH)3 extends on 5 orders of magnitude (from 9 to 4).[7] Greenwood and Earnshawn (1997)[8] refer to a pK value of 5.15, while a pK value of 3.80 is also reported in the Vogel's book.[9]
The formation of the complex (more exactly, in fact an ester) between one B(OH)3 molecule and two mannitol (C6H14O6) molecules (sometimes referred as mannitoborate, the conjugated base of the mannitoboric acid, pKa = 3.80), releases three water molecules and one proton in water as follows:
- ⇌ + 3 H2O + H+
- (pKa ranging from 4 to 9, depending on the mannitol concentration)
The solution obtained after the complexation/esterification reaction – involving also the release of a proton, from there, the ancient name of mannitoboric acid – is then sufficiently acid to be titrated by a strong base as NaOH. The equivalence point can then be determined by potentiometric titration using an automated titrator in order to assay the borate content present in aqueous solution. This method is often used to determine the boron content in the water of the primary circuit of light-water reactor, in which boric acid is added as a neutron moderator to control the reactivity of the core.
Polymeric ions

At neutral pH boric acid undergoes condensation reactions to form polymeric oxyanions. Well-known polyborate anions include the triborate(1−), tetraborate(2−) and pentaborate(1−) anions. The condensation reaction for the formation of tetraborate(2−) is as follows:
- 2 B(OH)3 + 2 [B(OH)4]− ⇌ [B4O5(OH)4]2− + 5 H2O
The tetraborate anion (tetramer) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH− groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very common structural motif in polyborate ions.
The tetraborate anion occurs in the mineral borax (sodium tetraborate octahydrate) with the formula Na2[B4O5(OH)4]·8H2O. The borax chemical formula is also commonly written in a more compact notation as Na2B4O7·10H2O. Sodium borate can be obtained in high purity and so can be used to make a standard solution in titrimetric analysis.[10]
A number of metal borates are known. They are produced by treating boric acid or boron oxides with metal oxides. Examples hereafter include[1] linear chains of 2, 3 or 4 trigonal BO3 structural units, each sharing only one oxygen atom with adjacent unit(s):
- diborate [B2O5]4−, found in Mg2B2O5 (suanite),
- triborate [B3O7]5−, found in CaAlB3O7 (johachidolite),
- tetraborate [B4O9]6−, found in Li6B4O9.
Metaborates, such as LiBO2, contain chains of trigonal BO3 structural units, each sharing two oxygen atoms with adjacent units, whereas NaBO2 and KBO2 contain the cyclic [B3O6]2− ion.[11]
Borosilicates
Borosilicate glass, also known as pyrex, can be viewed as a silicate in which some [SiO4]4− units are replaced by [BO4]5− centers, together with additional cations to compensate for the difference in valence states of Si(IV) and B(III). Because this substitution leads to imperfections, the material is slow to crystallise and forms a glass with low coefficient of thermal expansion, thus resistant to cracking when heated, unlike soda glass.
Minerals and uses

Common borate salts include sodium metaborate (NaBO2) and borax. Borax is soluble in water, so mineral deposits only occur in places with very low rainfall. Extensive deposits were found in Death Valley and shipped with twenty-mule teams from 1883 to 1889. In 1925, deposits were found at Boron, California on the edge of the Mojave Desert. The Atacama Desert in Chile also contains mineable borate concentrations.
Lithium metaborate, lithium tetraborate, or a mixture of both, can be used in borate fusion sample preparation of various samples for analysis by XRF, AAS, ICP-OES and ICP-MS. Borate fusion and energy dispersive X-ray fluorescence spectrometry with polarized excitation have been used in the analysis of contaminated soils.[12]
Disodium octaborate tetrahydrate (commonly abbreviated DOT) is used as a wood preservative or fungicide. Zinc borate is used as a flame retardant.
Borate esters
Borate esters are organic compounds, which are conveniently prepared by the stoichiometric condensation reaction of boric acid with alcohols.
Thin films
Metal borate thin films have been grown by a variety of techniques, including liquid-phase epitaxy (e.g. FeBO3,[13] β‐BaB2O4[14]), electron-beam evaporation (e.g. CrBO3,[15] β‐BaB2O4[16]), pulsed laser deposition (e.g. β‐BaB2O4,[17] Eu(BO2)3[18]), and atomic layer deposition (ALD). Growth by ALD was achieved using precursors composed of the tris(pyrazolyl)borate ligand and either ozone or water as the oxidant to deposit CaB2O4,[19] SrB2O4,[20] BaB2O4,[21] Mn3(BO3)2,[22] and CoB2O4[22] films.
References
- Wiberg E. and Holleman A.F. (2001) Inorganic Chemistry, Elsevier ISBN 0-12-352651-5
- Vegas, A. (1985). "New description of the Ca3(BO3)2 structure". Acta Crystallographica Section C. 41 (11): 1689–1690. doi:10.1107/S0108270185009052. ISSN 0108-2701.
- Ren, M.; Lin, J. H.; Dong, Y.; Yang, L. Q.; Su, M. Z.; You, L. P. (1999). "Structure and Phase Transition of GdBO3". Chemistry of Materials. 11 (6): 1576–1580. doi:10.1021/cm990022o. ISSN 0897-4756.
- Atkins; et al. (2010). Inorganic Chemistry (5th ed.). Oxford University Press. p. 334. ISBN 9780199236176.
- Ingri N. (1962) Acta Chem. Scand., 16, 439.
- Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, p. 357, ISBN 0-582-22628-7.
- NIST Special Publication. U.S. Government Printing Office. 1969.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, p. 357, ISBN 0-582-22628-7.
- Mendham, J.; Denney, R. C.; Barnes, J. D.; Thomas, M. J. K. (2000), Vogel's Quantitative Chemical Analysis (6th ed.), New York: Prentice Hall, p. 316, ISBN 0-582-22628-7.
- Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 205. ISBN 978-0-08-037941-8.
- Hettipathirana, Terrance D. (2004). "Simultaneous determination of parts-per-million level Cr, As, Cd and Pb, and major elements in low level contaminated soils using borate fusion and energy dispersive X-ray fluorescence spectrometry with polarized excitation". Spectrochimica Acta Part B: Atomic Spectroscopy. 59 (2): 223–229. Bibcode:2004AcSpe..59..223H. doi:10.1016/j.sab.2003.12.013.
- Yagupov, S.; Strugatsky, M.; Seleznyova, K.; Mogilenec, Yu.; Milyukova, E.; Maksimova, E.; Nauhatsky, I.; Drovosekov, A.; Kreines, N. (November 2016). "Iron borate films: Synthesis and characterization" (PDF). Journal of Magnetism and Magnetic Materials. 417: 338–343. Bibcode:2016JMMM..417..338Y. doi:10.1016/j.jmmm.2016.05.098.
- Liu, Junfang; He, Xiaoming; Xia, Changtai; Zhou, Guoqing; Zhou, Shengming; Xu, Jun; Yao, Wu; Qian, Liejia (July 2006). "Preparation of crystalline beta barium borate thin films on Sr2+-doped alpha barium borate substrates by liquid phase epitaxy". Thin Solid Films. 510 (1–2): 251–254. Bibcode:2006TSF...510..251L. doi:10.1016/j.tsf.2005.12.205.
- Jha, Menaka; Kshirsagar, Sachin D.; Ghanashyam Krishna, M.; Ganguli, Ashok K. (June 2011). "Growth and optical properties of chromium borate thin films". Solid State Sciences. 13 (6): 1334–1338. Bibcode:2011SSSci..13.1334J. doi:10.1016/j.solidstatesciences.2011.04.002.
- Maia, L. J. Q.; Feitosa, C. A. C.; De Vicente, F. S.; Mastelaro, V. R.; Li, M. Siu; Hernandes, A. C. (September 2004). "Structural and optical characterization of beta barium borate thin films grown by electron beam evaporation". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 22 (5): 2163–2167. Bibcode:2004JVST...22.2163M. doi:10.1116/1.1778409. ISSN 0734-2101.
- Xiao, R.‐F.; Ng, L. C.; Yu, P.; Wong, G. K. L. (1995-07-17). "Preparation of crystalline beta barium borate (β‐BaB2O4) thin films by pulsed laser deposition". Applied Physics Letters. 67 (3): 305–307. Bibcode:1995ApPhL..67..305X. doi:10.1063/1.115426. ISSN 0003-6951.
- Aleksandrovsky, A. S.; Krylov, A. S.; Potseluyko, A. M.; Seredkin, V. A.; Zaitsev, A. I.; Zamkov, A. V. (2006-02-09). Konov, Vitaly I.; Panchenko, Vladislav Y.; Sugioka, Koji; Veiko, Vadim P. (eds.). "Pulsed laser deposition of europium borate glass films and their optical and magneto-optical properties". Society of Photo-Optical Instrumentation Engineers (Spie) Conference Series. 6161: 61610A–61610A–7. Bibcode:2006SPIE.6161E..0AA. doi:10.1117/12.675020.
- Saly, Mark J.; Munnik, Frans; Winter, Charles H. (2010). "Atomic layer deposition of CaB2O4 films using bis(tris(pyrazolyl)borate)calcium as a highly thermally stable boron and calcium source". Journal of Materials Chemistry. 20 (44): 9995. doi:10.1039/c0jm02280b. ISSN 0959-9428.
- Saly, Mark J.; Munnik, Frans; Winter, Charles H. (June 2011). "The Atomic Layer Deposition of SrB2O4 Films Using the Thermally Stable Precursor Bis(tris(pyrazolyl)borate)strontium". Chemical Vapor Deposition. 17 (4–6): 128–134. doi:10.1002/cvde.201006890.
- Saly, Mark J.; Munnik, Frans; Baird, Ronald J.; Winter, Charles H. (2009-08-25). "Atomic Layer Deposition Growth of BaB2O4 Thin Films from an Exceptionally Thermally Stable Tris(pyrazolyl)borate-Based Precursor". Chemistry of Materials. 21 (16): 3742–3744. doi:10.1021/cm902030d. ISSN 0897-4756.
- Klesko, Joseph P.; Bellow, James A.; Saly, Mark J.; Winter, Charles H.; Julin, Jaakko; Sajavaara, Timo (September 2016). "Unusual stoichiometry control in the atomic layer deposition of manganese borate films from manganese bis(tris(pyrazolyl)borate) and ozone". Journal of Vacuum Science & Technology A: Vacuum, Surfaces, and Films. 34 (5): 051515. Bibcode:2016JVSTA..34e1515K. doi:10.1116/1.4961385. ISSN 0734-2101.
External links
| Wikimedia Commons has media related to Borates. |