Bosseopentaenoic acid
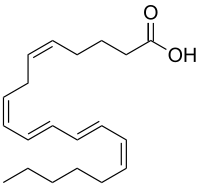
Bosseopentaenoic acid (BPA) is a conjugated polyunsaturated fatty acid. Bosseopentaenoic acid can be extracted from the red coralline algae, Bossiella orbigniana.[1] The first total synthesis of methyl bosseopentaenoate by consecutive palladium-catalyzed reactions was reported in 2011.[2] In 2017, bosseopentaenoic acid was obtained from the ester hydrolysis of methyl bosseopentaenoate in good yield using mild condition and the synthesis of its sulfur-bridged analogue of BPA; thiophene analogue was achieved by Mohamed and Solum [3] In this study, a comparison between the thiophene analogue and BPA with respect to their antioxidant activity was accomplished. It was shown that the rigidified analogue; the thiophene analogue exhibited higher free radical scavenging potential than the bosseopentaenoic acid. The results showed that by lowering the flexibility of the BPA as lead compound by incorporation thiophene ring in its structure, an increased antioxidant activity was observed. This study opens the door to investigate the relationship between the flexibility of other polyunsaturated fatty acids (PUFAs) and enhancement in the biological activity.
 | |
| Names | |
|---|---|
| IUPAC name
(5Z,8Z,10E,12E,14Z)-Eicosapentaenoic acid | |
| Other names
20:5Δ5Z,8Z,10E,12E,14Z | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.458 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Burgess, J.R.; De la Rosa, R.I.; Jacobs, R.S.; Butler, A (1991). "A new eicosapentaenoic acid formed from arachidonic acid in the coralline red algae Bossiella orbigniana". Lipids. 26 (2): 1057–1059. doi:10.1007/BF02544012. S2CID 43372222.
- Mohamed, Y. M. A.; Hansen, T. V. (2011). "Synthesis of methyl-(5Z,8Z,10E,12E,14Z)-eicosapentaenoate". Tetrahedron Letters. 52 (10): 1057–1059. doi:10.1016/j.tetlet.2010.12.078.
- Mohamed, Y. M. A.; Solum, E. J. (2017). "An efficient stereoselective synthesis of a sulfur-bridged analogue of bosseopentaenoic acid as a potential antioxidant agent". Arkivoc. 2017 (part v): 10–19. doi:10.24820/ark.5550190.p010.086.