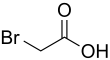
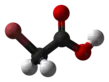
Bromoacetic acid
Bromoacetic acid is the chemical compound with the formula CH2BrCO2H. This colorless solid is a relatively strong alkylating agent. Bromoacetic acid and its esters are widely used building blocks in organic synthesis, for example in pharmaceutical chemistry.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bromoacetic acid | |||
| Other names
2-Bromoacetic acid Bromoethanoic acid α-Bromoacetic acid Monobromoacetic acid Carboxymethyl bromide UN 1938 | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 506167 | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.001.069 | ||
| EC Number |
| ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C2H3BrO2 | |||
| Molar mass | 138.948 g·mol−1 | ||
| Appearance | White to light yellow crystalline solid | ||
| Density | 1.934 g/mL | ||
| Melting point | 49 to 51 °C (120 to 124 °F; 322 to 324 K) | ||
| Boiling point | 206 to 208 °C (403 to 406 °F; 479 to 481 K) | ||
| polar organic solvents | |||
| Acidity (pKa) | 2.86[1] | ||
Refractive index (nD) |
1.4804 (50 °C, D) | ||
| Structure | |||
| Hexagonal or orthorhombic | |||
| Hazards[2] | |||
| GHS pictograms |     | ||
| GHS Signal word | Danger | ||
| H301, H311, H314, H317, H331, H400 | |||
| P260, P261, P264, P270, P271, P272, P273, P280, P301+310, P301+330+331, P302+352, P303+361+353, P304+340, P305+351+338, P310, P311, P312, P321, P322, P330, P333+313, P361, P363, P391, P403+233 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 110 °C (230 °F; 383 K) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
The compound is prepared by bromination of acetic acid, such as by a Hell–Volhard–Zelinsky reaction[3] or using other reagents.[4]
- CH3CO2H + Br2 → CH2BrCO2H + HBr
References
- Dippy, J.F.J., Hughes, S.R.C., Rozanski, A., J. Chem Soc., 1959, 2492.
- "Bromoacetic acid". pubchem.ncbi.nlm.nih.gov.
- Dagani, M. J.; Barda, H. J.; Benya, T. J.; Sanders, D. C. "Bromine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_405.
- Natelson, S.; Gottfried, S. (1955). "Ethyl Bromoacetate". Organic Syntheses.; Collective Volume, 3, p. 381
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.