Carnitine biosynthesis
Carnitine biosynthesis is a method for the endogenous production of L-carnitine, a molecule that is essential for energy metabolism.[1][2][3][4] In humans and many other animals, L-carnitine is obtained from both diet and by biosynthesis.[5][6] The carnitine biosynthesis pathway is highly conserved among many eukaryotes and some prokaryotes.[7][8][9]
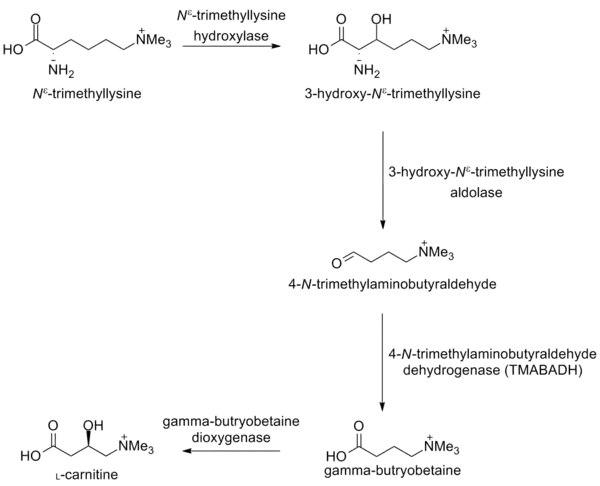
L-Carnitine is biosynthesized from Nε-trimethyllysine.[10] At least four enzymes are involved in the overall biosynthetic pathway. They are Nε-trimethyllysine hydroxylase, 3-hydroxy-Nε-trimethyllysine aldolase, 4-N-trimethylaminobutyraldehyde dehydrogenase and γ-butyrobetaine hydroxylase.
Nε-Trimethyllysine hydroxylase
The first enzyme of the L-carnitine biosynthetic pathway is Nε-trimethyllysine hydroxylase, an iron and 2-oxoglutarate (2OG)-dependent oxygenase that also requires ascorbate. [11] Nε-trimethyllysine hydroxylase catalyses the hydroxylation reaction of Nε-trimethyllysine to 3-hydroxy-Nε-trimethyllysine.
The current consensus theory about the origin of Nε-trimethyllysine in mammals is that mammals utilise lysosomal or proteasomal degradation of proteins containing Nε-trimethyllysine residues as starting point for carnitine biosynthesis.[12][13][14] An alternative theory involving endogenous non-peptidyl biosynthesis was also proposed, based on evidence gathered from a study involving feeding normal and undernourished human subjects with the amino acid lysine.[15] Although Nε-trimethyllysine biosynthetic pathway involving Nε-trimethyllysine methyltransferase has been fully characterised in fungi including Neurospora crassa, such biosynthetic pathway has never been properly characterised in mammals or humans.[16] A third theory about the origin of Nε-trimethyllysine in mammals does not involve biosynthesis at all, but involves direct dietary intake from vegetable foods. High-performance liquid chromatography (HPLC) analysis has confirmed that vegetables contains a significant amount of Nε-trimethyllysine.[17]
3-Hydroxy-Nε-trimethyllysine aldolase
The second step of L-carnitine biosynthesis requires the 3-hydroxy-Nε-trimethyllysine aldolase enzyme. 3-hydroxy-Nε-trimethyllysine aldolase is a pyridoxal phosphate dependent aldolase, and it catalyses the cleavage of 3-hydroxy-Nε-trimethyllysine into 4-N-trimethylaminobutyraldehyde and glycine.
The true identity of 3-hydroxy-Nε-trimethyllysine aldolase is elusive and the mammalian gene encoding 3-hydroxy-Nε-trimethyllysine aldolase has not been identified. 3-hydroxy-Nε-trimethyllysine aldolase activity has been demonstrated in both L-threonine aldolase and serine hydroxymethyltransferase,[18][19] although whether this is the main catalytic activity of these enzymes remains to be established.
4-N-Trimethylaminobutyraldehyde dehydrogenase
The third enzyme of L-carnitine biosynthesis is 4-N-trimethylaminobutyraldehyde dehydrogenase.[20] 4-N-trimethylaminobutyraldehyde dehydrogenase is a NAD+ dependent enzyme. 4-N-trimethylaminobutyraldehyde dehydrogenase catalyses the dehydrogenation of 4-N-trimethylaminobutyraldehyde into gamma-butyrobetaine.
Unlike 3-hydroxy-Nε-trimethyllysine aldolase, 4-N-trimethylaminobutyraldehyde dehydrogenase has been identified and purified from many sources including rat[21] and Pseudomonas.[22] However, the human 4-N-trimethylaminobutyraldehyde dehydrogenase has so far not been identified. There is considerable sequence similarity between rat 4-N-trimethylaminobutyraldehyde dehydrogenase and human aldehyde dehydrogenase 9,[23] but the true identity of 4-N-trimethylaminobutyraldehyde dehydrogenase remains to be established.
γ-Butyrobetaine hydroxylase
The final step of L-carnitine biosynthesis is γ-butyrobetaine hydroxylase, a zinc binding enzyme.[24][25][26][27][28][29] Like Nε-trimethyllysine hydroxylase, γ-butyrobetaine hydroxylase is a 2-oxoglutarate and iron(II)-dependent oxygenase. γ-Butyrobetaine hydroxylase catalyses the stereospecific hydroxylation of γ-butyrobetaine to L-carnitine.
γ-Butyrobetaine hydroxylase is the most studied enzyme among the four enzymes in the biosynthetic pathway. It has been purified from many sources, such as Pseudomonas,[30] rat,[31][32][33] cow,[34] guinea pig[35] and human.[36] Recombinant human γ-butyrobetaine hydroxylase has also been produced by Escherichia coli[27] and baculoviruses[26] systems.
References
- Activation and Transportation of Fatty Acids for Metabolism via Carnitine Shuttle Pathway (with Animation)
- Fraenkel, G.; Friedman, S. Carnitine. Vitam. Horm. 1957, 15, 73–118.
- Bremer, J. Carnitine – metabolism and functions. Physiol. Rev. 1983, 63, 1420–1480.
- Steiber, A.; Kerner, J.; Hoppel, C. L. Carnitine: a nutritional, biosynthetic, and functional perspective. Mol. Aspects Med. 2004, 25, 455–473.
- Rebouche, C. J. Carnitine function and requirements during the life cycle. FASEB J. 1992, 6, 3379–3386.
- Lennon, D. L.; Shrago, E. R.; Madden, M.; Nagle, F. J.; Hanson, P. Dietary carnitine intake related to skeletal muscle and plasma carnitine concentrations in adult men and women. Am. J. Clin. Nutr. 1986, 43, 234–238.
- Lindstedt, G.; Lindstedt, S.; Midtvedt, T.; Tofft, M. The formation and degradation of carnitine in Pseudomonas. Biochemistry 1967, 6, 1262–1270.
- Vaz, F. M.; Wanders, R. J. A. Carnitine biosynthesis in mammals. Biochem. J. 2002, 361, 417–429.
- Strijbis, K.; Vaz, F, M.; Distel, B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life 2010, 62, 357–362.
- Hulse, J. D.; Ellis, S. R.; Henderson, L. M. Carnitine biosynthesis. β-Hydroxylation of trimethyllysine by an α-ketoglutarate-dependent mitochondrial dioxygenase. J. Biol. Chem. 1978, 253, 1654–1659.
- Vaz, F. M.; Ofman, R.; Westinga, K.; Back, J. W.; Wanders, R. J. A. Molecular and biochemical characterization of rat ε-N-trimethyllysine hydroxylase, the first enzyme of carnitine biosynthesis. J. Biol. Chem. 2001, 276, 33512–33517.
- Bremer, J. Biosynthesis of carnitine in vivo. Biochim. Biophys. Acta 1961, 48, 622–624.
- Wolf, G.; Berger, C. R. A. Studies on the biosynthesis and turnover of carnitine. Arch. Biochem. Biophys. 1961, 92, 360–365.
- Paik, W. K.; Nochumson, S.; Kim, S. Carnitine biosynthesis via protein methylation. Trends Biochem. Sci. 1977, 2, 159–161.
- Khan-Siddiqui, L.; Bamji, M. S. Lysine-carnitine conversion in normal and undernourished adult man – suggestion of a nonpeptidyl pathway. Am. J. Clin. Nutr. 1983, 37, 93–98.
- Rebouche, C. J.; Broquist, H. P. Carnitine biosynthesis in Neurospora crassa: enzymatic conversion of lysine to ε-N-trimethyllysine. J. Bacteriol. 1976, 126, 1207–1214.
- Servillo, L.; Giovane, A.; Cautela, D.; Castaldo, D.; Balestrieri, M. L. Where Does Nε-Trimethyllysine for the Carnitine Biosynthesis in Mammals Come from? PLoS ONE 2014, 9, e84589.
- McNeil, J. B.; Flynn, J.; Tsao, N.; Monschau, N.; Stahmann, K. P.; Haynes, R. H.; McIntosh, E. M.; Pearlman, R. E. Glycine metabolism in Candida albicans: characterization of the serine hydroxymethyltransferase (SHM1, SHM2) and threonine aldolase (GLY1) genes. Yeast 2000, 16, 167–175.
- Schirch, L.; Peterson, D. Purification and properties of mitochondrial serine hydroxymethyltransferas. J. Biol. Chem. 1980, 255, 7801–7806.
- Hulse, J. D.; Henderson, L. M. Carnitine biosynthesis. Purification of 4-N’-trimethylaminobutyraldehyde dehydrogenase from beef liver. J. Biol. Chem. 1980, 255, 1146–1151.
- Vaz, F. M.; Fouchier, S. W.; Ofman, R. ; Sommer, M.; Wanders, R. J. A. Molecular and biochemical characterization of rat γ-trimethylaminobutyraldehyde dehydrogenase and evidence for the involvement of human aldehyde dehydrogenase 9 in carnitine biosynthesis. J. Biol. Chem. 2000, 275, 7390–7394.
- Hassan, M.; Okada, M.; Ichiyanagi, T.; Mori, N. 4-N-Trimethylaminobutyraldehyde dehydrogenase: purification and characterization of an enzyme from Pseudomonas sp. 13CM. Biosci. Biotechnol. Biochem. 2008, 72, 155–162.
- Lin, S. W.; Chen, J. C.; Hsu, L. C.; Hsieh, C. L.; Yoshida, A. Human γ-aminobutyraldehyde dehydrogenase (ALDH9): cDNA sequence, genomic organization, polymorphism, chromosomal localization, and tissue expression. Genomics 1996, 34, 376–380
- Vaz, F. M.; van Gool, S.; Ofman, R.; Ijlst, L.; Wanders, R. J. Carnitine Biosynthesis: Identification of the cDNA Encoding Human γ-Butyrobetaine Hydroxylase. Biochem. Biophys. Res. Commun. 1998, 250, 506–510.
- Rigault, C.; Le Borgne, F.; Demarquoy, J. Genomic structure, alternative maturation and tissue expression of the human BBOX1 gene. Biochim. Biophys. Acta 2006, 1761, 1469–1481.
- Leung, I. K. H.; Krojer, T. J.; Kochan, G. T.; Henry, L.; von Delft, F.; Claridge, T. D. W.; Oppermann, U.; McDonough, M. A.; Schofield, C. J. Structural and mechanistic studies on γ-butyrobetaine hydroxylase. Chem. Biol. 2010, 17, 1316–1324.
- Tars, K.; Rumnieks, J.; Zeltins, A.; Kazaks, A.; Kotelovica, S.; Leonciks, A.; Saripo, J.; Viksna, A.; Kuka, J.; Liepinsh, E.; Dambrova, M. Crystal structure of human gamma-butyrobetaine hydroxylase. Biochem. Biophys. Res. Commun. 2010, 298, 634–639.
- Lindstedt, G.; Lindstedt, S.; Olander, B.; Tofft, M. α-ketoglutarate and hydroxylation of γ-butyrobetaine. Biochim. Biophys. Acta 1968, 158, 503–505.
- Lindstedt, G.; Lindstedt, S. Cofactor requirements of γ-butyrobetaine hydroxylase from rat liver. J. Biol. Chem. 1970, 245, 4178–4186.
- Lindstedt, G.; Lindstedt, S.; Tofft, S. γ-Butyrobetaine hydroxylase from Pseudomonas sp AK 1. Biochemistry 1970, 9, 4336–4342
- Lindstedt, G. Hydroxylation of γ-butyrobetaine to carnitine in rat liver. Biochemistry 1967, 6, 1271–1282.
- Paul, H. S.; Sekas, G.; Adibi, S. A. Carnitine biosynthesis in hepatic peroxisomes. Demonstration of γ-butyrobetaine hydroxylase activity. Eur. J. Chem. 1992, 203, 599–605.
- Galland, S.; Leborgne, F.; Guyonnet, D.; Clouet, P.; Demarquoy, J. Purification and characterization of the rat liver γ-butyrobetaine hydroxylase. Mol. Cell. Biochem. 1998, 178, 163–168.
- Kondo, A.; Blanchard, J. S.; Englard, S. Purification and properties of calf liver γ-butyrobetaine hydroxylase. Arch. Biochem. Biophys. 1981, 212, 338–346.
- Dunn. W. A.; Rettura, G.; Seifter, E.; Englard, S. Carnitine biosynthesis from γ-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. J. Biol. Chem. 1984, 259, 10764–10770.
- Lindstedt, G.; Lindstedt, S.; Nordin, I. γ-Butyrobetaine hydroxylase in human kidney. Scand. J. Clin. Lab. Invest. 1982, 42, 477–485.