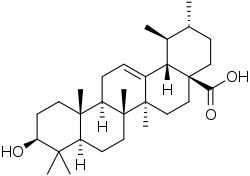
Corosolic acid
Corosolic acid is a pentacyclic triterpene acid found in Lagerstroemia speciosa. It is similar in structure to ursolic acid, differing only in the fact that it has a 2-alpha-hydroxy attachment.[1]
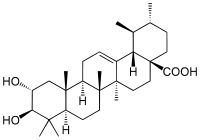 | |
| Names | |
|---|---|
| IUPAC name
(1S,2R,4aS,6aR,6aS,6bR,8aR,10R,11R,12aR,14bS)-10,11-Dihydroxy-1,2,6a,6b,9,9,12a-heptamethyl-2,3,4,5,6,6a,7,8,8a,10,11,12,13,14b-tetradecahydro-1H-picene-4a-carboxylic acid | |
| Other names
Glucosol; Corsolic acid; Colosic acid; 2α-Hydroxyursolic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.125.730 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C30H48O4 | |
| Molar mass | 472.710 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- "Asiatic Acid, Corosolic Acid, and Maslinic Acid Interfere with Intracellular Trafficking and N-Linked Glycosylation of Intercellular Adhesion Molecule-1". doi:10.1248/bpb.b18-00276. Cite journal requires
|journal=(help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.