Cyclobutanone
Cyclobutanone is an organic compound with molecular formula (CH2)3CO. It is a four-membered cyclic ketone (cycloalkanone). It is a colorless volatile liquid at room temperature. Since cyclopropanone is highly sensitive, cyclobutanone is the smallest, easily handled cyclic ketone.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.405 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H6O | |
| Molar mass | 70.091 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 0.9547 g/cm3 (0 °C)[1] |
| Melting point | −50.9 °C (−59.6 °F; 222.2 K)[1] |
| Boiling point | 99.75 °C (211.55 °F; 372.90 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Preparation
The Russian chemist Nikolai Kischner first prepared cyclobutanone in 1905.[2][3] He synthesized cyclobutanone in a low yield from cyclobutanecarboxylic acid in several reaction steps. This process is cumbersome and inefficient by today's standards.
 Synthesis of cyclobutanone from cyclobutanecarboxylic acid
Synthesis of cyclobutanone from cyclobutanecarboxylic acid
More efficient, high-yielding syntheses have since been developed.[4] One strategy involves degradation of five-carbon building blocks. For example, the oxidative decarboxylation of cyclobutanecarboxylic acid was improved by the use of other reagents and methods. A newer, more efficient preparation of cyclobutanone was found by P. Lipp and R. Köster in which a solution of diazomethane in diethyl ether is reacted with ketene.[5] This reaction is based on a ring expansion of the cyclopropanone intermediate initially formed, wherein molecular nitrogen is split off. The reaction mechanism was confirmed by a reaction using 14C-labeled diazomethane.[6]
 Preparation of cyclobutanone from diazomethane and ketene via cyclopropanone
Preparation of cyclobutanone from diazomethane and ketene via cyclopropanone
Another synthesis of cyclobutanone involves lithium-catalyzed rearrangement of oxaspiropentane, which is formed by epoxidation of the easily accessible methylenecyclopropane.[7][8]
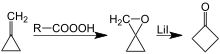 Preparation of cyclobutanone by rearrangement
Preparation of cyclobutanone by rearrangement
Cyclobutanone can also be prepared in a two step procedure by dialkylation of 1,3-dithiane with 1-bromo-3-chloropropane followed by deprotection to the ketone with mercuric chloride (HgCl2) and cadmium carbonate (CdCO3).[9]
Reactions
At about 350 °C, cyclobutanone decomposes into ethylene and ketene.[10] The activation energy for this [2+2] cycloreversion is 52 kcal/mol. The reversion reaction, the [2+2] cycloaddition of ketene and ethylene, has never been observed.
 Decomposition of cyclobutanone
Decomposition of cyclobutanone
References
- CRC Handbook of Chemistry and Physics. 90. Boca Raton, Florida: CRC Press.
- N. Kishner (1905). "'Über die Einwirkung von Brom auf die Amide α-bromsubstituierter Säuren". Journal der Russischen Physikalisch-Chemischen Gesellschaft. 37: 103–105.
- N. Kishner (1905). "Über das Cyklobutanon". Journal der Russischen Physikalisch-Chemischen Gesellschaft. 37: 106–109.
- Dieter Seebach (1971). "Isocyclische Vierringverbindungen". In Houben; Weyl; Müller (eds.). Methoden der Organischen Chemie. IV/4. Stuttgart: Georg Thieme Verlag.
- P. Lipp und R. Köster (1931). "Ein neuer Weg zum Cyclobutanon". Berichte der Deutschen Chemischen Gesellschaft. 64 (11): 2823–2825. doi:10.1002/cber.19310641112.
- Semenow, Dorothy A.; Cox, Eugene F.; Roberts, John D. (1956). "Small-Ring Compounds. XIV. Radioactive Cyclobutanone from Ketene and Diazomethane-14C1". Journal of the American Chemical Society. 78 (13): 3221–3223. doi:10.1021/ja01594a069.
- Salaün, J. R.; Conia, J. M. (1971). "Oxaspiropentane. A rapid route to cyclobutanone". Journal of the Chemical Society D: Chemical Communications (23): 1579b-1580. doi:10.1039/C2971001579B.
- J. R. Salaün, J. Champion, J. M. Conia (1977). "Cyclobutanone from Methylenecyclopropane via Oxaspiropentane". Organic Syntheses. 57: 36. doi:10.15227/orgsyn.057.0036.CS1 maint: multiple names: authors list (link); Collective Volume, 6, p. 320
- D. Seebach, A. K. Beck (1971). "Cyclic Ketones from 1,3-Dithiane: Cyclobutanone". Organic Syntheses. 51: 76. doi:10.15227/orgsyn.051.0076.; Collective Volume, 6, p. 316
- Das, M. N.; Kern, F.; Coyle, T. D.; Walters, W. D. (1954). "The Thermal Decomposition of Cyclobutanone1". Journal of the American Chemical Society. 76 (24): 6271–6274. doi:10.1021/ja01653a013.