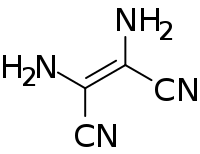
Diaminomaleonitrile
Diaminomaleonitrile (DAMN) is an organic compound composed of two amino groups and two nitrile groups bonded to a central alkene unit. The systematic name reflects its relationship to maleic acid.
 | |
| Names | |
|---|---|
| IUPAC name
(Z)-2,3-Diaminobut-2-enedinitrile | |
| Other names
2,3-Diaminomaleonitrile; Hydrogen cyanide tetramer | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.013.361 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H4N4 | |
| Molar mass | 108.104 g·mol−1 |
| Melting point | 178–179 °C (352–354 °F; 451–452 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The chemical can be formed by polymerization of hydrogen cyanide and can be used as the starting point for the synthesis of several classes of heterocyclic compounds. Therefore, it has been considered as a possible organic chemical present in prebiotic conditions.[2]
References
- "Diaminomaleonitrile". Sigma-Aldrich.
- Al-Azmi, A.; Elassar, A.-Z. A.; Booth, B. L. (2003). "The Chemistry of Diaminomaleonitrile and its Utility in Heterocyclic Synthesis". Tetrahedron. 59 (16): 2749–2763. doi:10.1016/S0040-4020(03)00153-4.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.