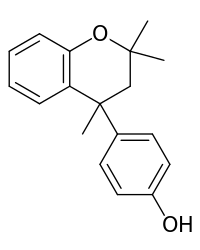
Dianin's compound
Dianin's compound (4-p-hydroxyphenyl-2,2,4-trimethylchroman) was first prepared by Aleksandr Dianin in 1914.[1] This compound is a condensation isomer of bisphenol A and acetone and of special importance in host–guest chemistry because it can form a large variety of clathrates with suitable guest molecules. One example is the clathrate of Dianin's compound with morpholine.[2] Slow evaporation of a solution containing both organic compounds yields crystals. Each asymmetric unit cell making up the crystal contains six chroman molecules of which two are deprotonated and two protonated morpholine molecules. The six chroman molecules are racemate pairs.
 | |
| Names | |
|---|---|
| IUPAC name
4-(2,2,4-Trimethyl-4-chromanyl)phenol | |
| Other names
4-p-Hydroxyphenyl-2,2,4-trimethylchroman | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.006.775 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H20O2 | |
| Molar mass | 268.35 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Dianin, A. P. (1914). "Condensation of phenol with unsaturated ketones. Condensation of phenol with mesityl oxide". Zhurnal Russkago Fiziko-Khimicheskago Obshchestva (J. Russ. Phys. Chem. Soc.) (in Russian). 36: 1310–1319.
- Lloyd, Gareth O.; Bredenkamp, Martin W.; Barbour, Leonard J. (2005). "Enclathration of morpholinium cations by Dianin's compound: Salt formation by partial host-to-guest proton transfer". Chemical Communications. 2005 (32): 4053–4055. doi:10.1039/b507726e. PMID 16091797.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.