Dihydrolipoic acid
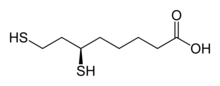
Dihydrolipoic acid is an organic compound that is the reduced form of lipoic acid. This carboxylic acid features a pair of thiol groups, and therefore is a dithiol. It is optically active, but only the R-enantiomer is biochemically significant. The lipoic acid/dihydrolipoic acid pair participate in a variety of biochemical transformations.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
6,8-Bis(sulfanyl)octanoic acid[1] | |
| Other names
6,8-Dimercaptooctanoic acid Reduced lipoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.120.390 |
| KEGG | |
| MeSH | Dihydrolipoic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H16O2S2 | |
| Molar mass | 208.33 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
See also
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 697. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
The prefixes ‘mercapto’ (–SH), and ‘hydroseleno’ or selenyl (–SeH), etc. are no longer recommended.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.