Dimethylphosphite
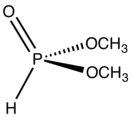
Dimethylphosphite is an organophosphorus compound with the formula (CH3O)2P(O)H. It is a reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. The molecule is tetrahedral. It is a colorless liquid. The compounds can be prepared by methanolysis of phosphorus trichloride or by heating diethylphosphite in methanol.[1]
 | |
| Names | |
|---|---|
| Other names
Phosphonic acid, dimethyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.011.622 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H7O3P | |
| Molar mass | 110.049 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.20 g/cm3 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
References
- Balint, Erika; Tajti, Adam; Drahos, Laszlo; Ilia, Gheorge; Keglevich, Gyorgy (2013). "Alcoholysis of Dialkyl Phosphites Under Microwave Conditions". Current Organic Chemistry. 17: 555–562.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.