Dissimilatory sulfate reduction
Dissimilatory sulfate reduction is a form of anaerobic respiration that uses sulfate as the terminal electron acceptor. This metabolism is found in some types of bacteria and archaea which are often termed sulfate-reducing organisms.
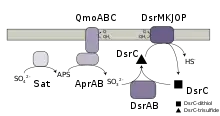
Dissimilatory sulfate reduction occurs in four steps:[1]
- Conversion (activation) of sulfate to Adenosine 5’-phosphosulfate (APS) via Sulfate adenylyltransferase
- reduction of APS to sulfite via Adenylyl-sulfate reductase
- transfer of the sulfur atom of sulfite to the DsrC protein creating a trisulfide intermediate catalyzed by DsrAB.
- reduction of the trisulfide to sulfide and reduced DsrC via a membrane bound enzyme, DsrMKJOP.
Which requires the consumption of a single ATP molecule and the input of 8 electrons (e−).[2][3]
The protein complexes responsible for these chemical conversions — Sat, Apr and Dsr — are found in all currently known organisms that perform dissimilatory sulfate reduction.[4] Energetically, sulfate is a poor electron acceptor for microorganisms as the sulfate-sulfite redox couple is E0' -516 mV, which is too negative to allow reduction by NADH or ferrodoxin that are the primary intracellular electron mediators.[5] To overcome this issue, sulfate is first converted into APS by the enzyme ATP sulfurylase (Sat), at the cost of a single ATP molecule. The APS-sulfite redox couple has a E0' of -60 mV, which allows APS to be reduced by either NADH or reduced ferrodoxin using the enzyme adenylyl-sulfate reductase (Apr), which requires the input of 2 electrons.[5] In the final step, sulfite is reduced by the dissimilatory sulfite reductase (Dsr) to form sulfide, requiring the input of 6 electrons.[3]
Note. The term "dissimilatory" is used when hydrogen sulfide is produced in an anaerobic respiration process. By contrast, the term "assimilatory" would be used in relation to the biosynthesis of organo-sulfur compounds.
See also
References
- Santos, AA; Venceslau, SS; Grein, F; Leavitt, WD; Dahl, C; Johnston, DT; Pereira, IA (18 December 2015). "A protein trisulfide couples dissimilatory sulfate reduction to energy conservation". Science. 350 (6267): 1541–5. doi:10.1126/science.aad3558. PMID 26680199.
- Barton, Larry L.; Fardeau, Marie-Laure; Fauque, Guy D. (2014). "Chapter 10. Hydrogen Sulfide: A Toxic Gas Produced by Dissimilatory Sulfate and Sulfur Reduction and Consumed by Microbial Oxidation". In Peter M.H. Kroneck and Martha E. Sosa Torres (ed.). The Metal-Driven Biogeochemistry of Gaseous Compounds in the Environment. Metal Ions in Life Sciences. 14. Springer. pp. 237–277. doi:10.1007/978-94-017-9269-1_10.
- Grein F, Ramos AR, Venceslau SS, Pereira IA (February 2013). "Unifying concepts in anaerobic respiration: insights from dissimilatory sulfur metabolism". Biochim. Biophys. Acta. 1827 (2): 145–60. doi:10.1016/j.bbabio.2012.09.001. PMID 22982583.
- Pereira IA, Ramos AR, Grein F, Marques MC, da Silva SM, Venceslau SS (2011). "A comparative genomic analysis of energy metabolism in sulfate reducing bacteria and archaea". Front Microbiol. 2: 69. doi:10.3389/fmicb.2011.00069. PMC 3119410. PMID 21747791.
- Muyzer G, Stams AJ (June 2008). "The ecology and biotechnology of sulphate-reducing bacteria". Nat. Rev. Microbiol. 6 (6): 441–54. doi:10.1038/nrmicro1892. PMID 18461075.