Dithiobiuret
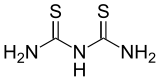
Dithiobiuret is an organosulfur compound with the formula HN(C(S)NH2)2. It is a colourless solid that is soluble in warm water and polar organic solvents. It is a planar molecule with short C-S and C-N distances (1.69, 1.38 Å, resp.) indicative of multiple C-S and C-N bonding.[1]
 | |
| Names | |
|---|---|
| IUPAC name
Dicarbonodithioimidic diamide, dithioallophanimidic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.987 |
| EC Number |
|
| MeSH | 2,4-dithiobiuret |
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 2811 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H5N3S2 | |
| Molar mass | 135.20 g·mol−1 |
| Appearance | White crystals |
| Density | 1.54 g/cm3 |
| log P | −0.415 |
| Acidity (pKa) | 11.152 |
| Basicity (pKb) | 2.845 |
| Hazards | |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H300, H310, H330 | |
| P260, P280, P284, P302+350, P310 | |
| Related compounds | |
Related compounds |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The compound can be viewed as the product from the condensation of two molecules of thiourea, but it is prepared by treatment of 2-cyanoguanidine with hydrogen sulfide. The conversion proceeds via guanylthiourea:
- NCNC(NH2) + H2S → HN(C(S)NH2)(C(NH)NH2)
- HN(C(S)NH2)(C(NH)NH2) + H2S → HN(C(S)NH2)2
It is used as a plasticizer, a rubber accelerator, and as an intermediate in pesticide manufacturing.[2] It is extremely toxic; exposure can result in respiratory failure.
See also
References
- Spofford, W. A.; Amma, E. L. (1972). "Crystal and molecular structure of dithiobiuret". Journal of Crystal and Molecular Structure. 2 (4): 151–158. doi:10.1007/BF01275491.
- Dithiobiuret Hazardous Substance Fact Sheet, New Jersey Department of Health and Senior Services
External links
- Williams, KD; Porter, WR; Peterson, RE (1982). "Dithiobiuret metabolism in the rat". Neurotoxicology. 3 (4): 221–31. PMID 6820683.
- Dithiobiuret at www.chemicalbook.com.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.