Fukuyama indole synthesis
The Fukuyama indole synthesis is a versatile tin mediated chemical reaction that results in the formation of 2,3-disubstituted indoles.[1] A practical one-pot reaction that can be useful for the creation of disubstituted indoles.[2] Most commonly tributyltin hydride is utilized as the reducing agent, with azobisisobutyronitrile (AIBN) as a radical initiator. Triethylborane can also be used as a radical initiator.[3] The reaction can begin with either an ortho-isocyanostyrene or a 2-alkenylthioanilide derivative, both forming the indole through Radical cyclization via an α-stannoimidoyl radical.[4] The R group can be a range of both basic and acidic sensitive functional groups such as esters, THP ethers, and β-lactams. In addition the reaction is not stereospecific, in that both the cis and trans isoform can be used to obtain the desired product.[5]
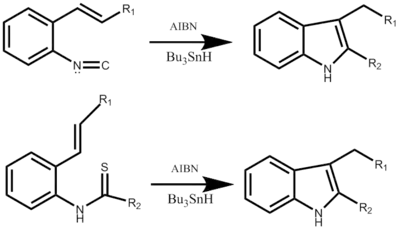
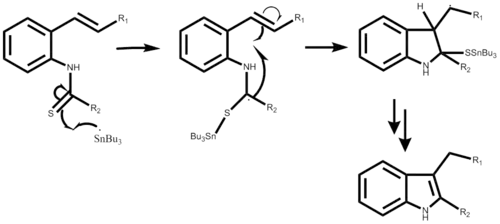
Mechanism
The reaction mechanism begins with the creation of the tributyl tin radical with either AIBN or triethylborane, not shown in either step-wise mechanism. Following the radical attacks the o-isocyano carbon creating the alpha-stannoimidoyl radical. Through radical cyclization a five membered ring is formed followed by the propagation of a new tin radical. The final step is dependent on the desired outcome of the reaction. This reaction is a one-pot synthesis and results in yields ranging from 50% to 98% depending on the substituent.[1]

The mechanism using 2-alkenylthioanilide is very similar, also starting with the formation of a bond, now between the tin radical and the sulfur. Followed by a similar radical cyclization resulting in a five membered ring, a new tin radical is produced and the original attacking radical leaves with the sulfur substituent. This part of the step-wise mechanism has yet to be detailed. The reaction yield can range from 40% to 93% depending also on the desired substituent.
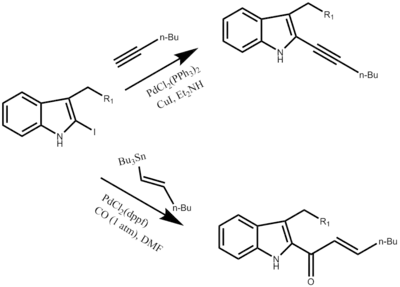
Derivatives
The Fukuyama Indole synthesis can generate a range of different substituents at the 2,3 position that were previously unattainable without a protecting group on the nitrogen in the ring. One such example is the 2-iodoindole derivative, which can then lead to a variety of N-unprotected 2,3 substituted indoles. Before the discovery of this compound the chemistry involving 2-stannylindoles was not developed as there was no way to practically synthesis these N-unprotected 2,3-stannylindoles. One was limited to the production of N-protected 2-stannylindoles through metalation by a process known as Stille coupling.[6] The N-unprotected 2-stannylindoles generated from the Fukuyama Synthesis can be readily oxidized with iodine opening up an area of chemistry that allows for the synthesis of a variety compounds utilizing the 2-iodoindoles as a starting reagent. This iodine substituted derivative can lead to aryl halides, vinyl iodides, vinyl triflates, benzyl bromides.

In addition to acetylenes (Sonogashira coupling), and acrylates (Heck reaction) in the second position.[5]
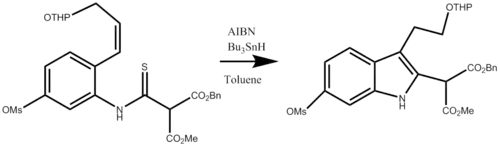
Applications
The synthesis is one of the simplest methods for creating poly-substituted indoles, this procedure has been utilized in numerous natural product syntheses, including aspidophytine,[7] vinblastine,[8] and strychnine[9]
Shown below is the fourth step in the synthesis of (+)-Vinblastine, the application of the Fukuyama Indole synthesis to create a disubstituted indole.
In addition, the fukuyama reaction plays a role in the syntheses of indolocarbazoles,[5] biindolyls,[5] and the total synthesis of vincadifformine and tabersonine.[10]
References
- Fukuyama, T.; Chen, X.; Peng, G. (1994). "A Novel Tin-Mediated Indole Synthesis". J. Am. Chem. Soc. 116 (7): 3127–8. doi:10.1021/ja983681v.
- Pindur, U.; Adam, R. (1998). "Synthetically attractive indolization processes and newer methods for the preparation of selectively substituted indoles". J. Heterocycl. Chem. 25 (1): 1–8. doi:10.1002/jhet.5570250101.
- Tokuyama, H.; Yamashita, T.; Reding, M. T.; Kaburagi, Y.; Fukuyama, T. (1999). "Radical Cyclization of 2-Alkenylthioanilides: A Novel Synthesis of 2,3-Disubstituted Indoles". J. Am. Chem. Soc. 121 (15): 3791–2. doi:10.1021/ja983681v.
- Gribble, G. (2000). "Recent developments in indole ring synthesis—methodology and applications". J. Chem. Soc. Perkin Trans. 1. 2000 (7): 1045–75. doi:10.1039/a909834h.
- Kobayashi, T.; Fukuyama, T. (1998). "Development of a novel indole synthesis". J. Heterocycl. Chem. 35 (5): 1043–56. doi:10.1002/jhet.5570350504.
- Trost, B. M.; Fortunak, J. M. (1982). "Cyclizations initiated by a Pd2+-Ag+ mixed-metal system". Organometallics. 1 (7): 7–10. doi:10.1021/om00061a003.
- Sumi, S.; Matsumoto, K.; Tokuyama, H.; Fukuyama, T. (2003). "Enantioselective Total Synthesis of Aspidophytine". Org. Lett. 5 (11): 1891–3. doi:10.1021/ol034445e. PMID 12762679.
- Yokoshima, S.; Ueda, T.; Kobayashi, S.; Sato, A.; Kuboyama, T.; Tokuyama, H.; Fukuyama, T. (2002). "Stereocontrolled Total Synthesis of (+)-Vinblastine". J. Am. Chem. Soc. 124 (10): 2137–9. CiteSeerX 10.1.1.414.6638. doi:10.1021/ja0177049. PMID 11878966.
- Kaburagi, Y.; Tokuyama, H.; Fukuyama, T. (2004). "Total Synthesis of (−)-Strychnine". J. Am. Chem. Soc. 126 (33): 10246–7. doi:10.1021/ja046407b. PMID 15315428.
- Kobayashi, S.; Peng, G.; Fukuyama, T. (1999). "Efficient total syntheses of (±)-vincadifformine and (−)-tabersonine". Tetrahedron Lett. 40 (8): 1519–22. doi:10.1016/S0040-4039(98)02667-7.