Fulvenes
Fulvenes are the class of hydrocarbon obtained by formally cross-conjugating one ring and methylidene through a common exocyclic double bond.[1][2]
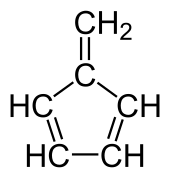
Chemical structure of fulvene
The name is derived from fulvene, which has one pentagonal ring. Other examples include methylenecyclopropene (triafulvene) and heptafulvene.
References
- Agranat, Israel (2012), "Ground-State Versus Excited-State Polarity of Triafulvenes: A Study of Solvent Effects on Molecular Electronic Spectra", The Jerusalem Symposia on Quantum Chemistry and Biochemistry, 8: 573–583, doi:10.1007/978-94-010-1837-1_36
- Neuenschwander, Markus (1986), "Synthetic and NMR spectroscopic investigations of fulvenes and fulvalenes" (PDF), Pure Appl. Chem., 58 (1): 55–66, doi:10.1351/pac198658010055
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.