Furfurylamine
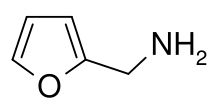
Furfurylamine is an aromatic amine typically formed by the reductive amination of furfural with ammonia.
 | |
| Names | |
|---|---|
| IUPAC name
1-(2-Furyl)methylamine | |
| Other names
furfurylamine, 2-Aminomethylfuran | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.580 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2526 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H7NO | |
| Molar mass | 97.117 g·mol−1 |
| Density | 1.099 g/mL liquid |
| Melting point | −70 °C (−94 °F; 203 K) |
| Boiling point | 145 °C (293 °F; 418 K) |
| Soluble | |
| Hazards | |
| GHS pictograms |     |
| GHS Signal word | Danger |
| H226, H301, H302, H310, H311, H312, H314, H332 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P270, P271, P280, P301+310, P301+312, P301+330+331, P302+350, P302+352, P303+361+353, P304+312, P304+340, P305+351+338, P310, P312, P321 | |
| Flash point | 37 °C (99 °F; 310 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The pharmaceutical drug furtrethonium, a parasympathomimetic cholinergic, is a trimethyl ammonium derivative of furfurylamine.
Furfurylamine also has use in the synthesis of Barmastine.
See also
- 2-Furonitrile - corresponding nitrile
- Furan-2-ylmethanethiol - corresponding thiol
- Furfuryl alcohol - corresponding alcohol
- 2-Furoic acid - corresponding carboxylic acid
References
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.