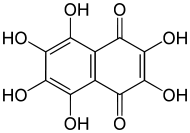
Hexahydroxy-1,4-naphthalenedione
2,3,5,6,7,8-Hexahydroxy-1,4-naphthalenedione, also called hexahydroxynaphthoquinone or spinochrome E,[1] is an organic compound with formula C
10H
6O
8. It is formally derived from naphthoquinone (1,4-naphtalenedione) through replacement of all six hydrogen atoms by hydroxyl (OH) groups. The numerical prefixes "2,3,5,6,7,8" are superfluous, since there is no other hexahydroxy derivative of 1,4-naphthoquinone.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Hexahydroxy-1,4-naphthalenedione | |
| Systematic IUPAC name
Hexahydroxy-1,4-dihydronaphthalene-1,4-dione | |
| Other names
Hexahydroxynaphthalene-1,4-dione; Spinochrome E | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H6O8 | |
| Molar mass | 254.15 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The substance forms red micro-needles which do not melt below 300 °C, and can be sublimed in vacuum at about 265 °C.
The compound occurs in the shell ("test") and spines of the sea urchins Paracentrotus lividus and Psammechinus miliaris.
The compound can be produced by condensation of 3,4,5,6-tetramethoxyphthalaldehyde with glyoxal.[2]
See also
- Hexahydroxy-2,3-naphthalenedione, a structural isomer.
- Tetrahydroxybenzoquinone
- Octahydroxyanthraquinone
References
- T. W. Goodwin, E. Lederer and L. Musajo (1951), The nomenclature of the spinochromes of sea urchins. Cellular and Molecular Life Sciences, Volume 7, Number 10, pages 375-376. doi:10.1007/BF02168905
- H. A. Anderson and R. H. Thomson (1966), Naturally Occurring Quinones. Part VIP Synthesis of Spinochrome E. J. Chem. Soc. series C (Organic), pages 426-428. doi:10.1039/J39660000426
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.