Indolizine
Indolizine (Chemical formula C8H7N) is a heterocyclic aromatic organic compound that is an isomer of indole. The saturated analog indolizidine forms the structural core of a variety of alkaloids such as swainsonine.
 | |
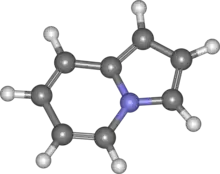 | |
| Names | |
|---|---|
| IUPAC name
Indolizine | |
| Other names
Pyrrocoline; Indolizin; Pyrrolo[1,2-a]pyridine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.219.195 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H7N | |
| Molar mass | 117.151 g·mol−1 |
| Appearance | White solid |
| Melting point | 75 °C (167 °F; 348 K) |
| Boiling point | 205 °C (401 °F; 478 K) |
| Basicity (pKb) | 10.1 [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Weight
The known molar mass of C8H7N is 117.151 g/mol.[2]
Properties
The following is a list of both physical and chemical properties:
References
- Elattar, K.M.; Youssef, I.; Fadda, A.A. (4 May 2016). "Reactivity of indolizines in organic synthesis". Synthetic Communications Reviews. 46 (9): 719–744. doi:10.1080/00397911.2016.1166252.
- "Indolizine".
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.