Isoguanine
Isoguanine or 2-hydroxyadenine is a purine base that is an isomer of guanine. It is a product of oxidative damage to DNA and has been shown to cause mutation.[1] It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[2][3]
 | |
| Names | |
|---|---|
| IUPAC name
6-Amino-1,7-dihydropurin-2-one | |
| Other names
2-Hydroxyadenine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.144 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H5N5O | |
| Molar mass | 151.1261 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
It is used as a nucleobase of hachimoji nucleic acids.[4] In hachimoji DNA, it pairs with 1-methylcytosine, while in hachimoji RNA, it pairs with isocytosine.
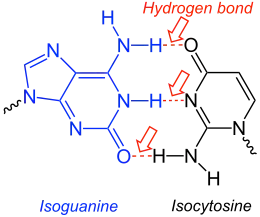
Isoguanine-Isocytosine-base-pair
References
- Yang XL, Sugiyama H, Ikeda S, Saito I, Wang AH (1998). "Structural studies of a stable parallel-stranded DNA duplex incorporating isoguanine:cytosine and isocytosine:guanine basepairs by nuclear magnetic resonance spectroscopy". Biophys. J. 75 (3): 1163–1171. doi:10.1016/S0006-3495(98)74035-4. PMC 1299791. PMID 9726918.
- Andrzej Jaworski, Józef S. Kwiatkowski, Bogdan Lesyng: „Why isoguanine and isocytosine are not the components of the genetic code", International Journal of Quantum Chemistry, Supplement: Proceedings of the International Symposium on Quantum Biology and Quantum Pharmacology, 1985, 28 (Supplement S12), pp. 209–216 (doi:10.1002/qua.560280720).
- Christopher Roberts, Rajanikanth Bandaru, Christopher Switzer: „Theoretical and Experimental Study of Isoguanine and Isocytosine: Base Pairing in an Expanded Genetic System", J. Am. Chem. Soc., 1997, 119 (20), pp. 4640–4649 (doi:10.1021/ja970123s).
- Hoshika, Shuichi; et al. (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science. 363 (6429): 884–887. doi:10.1126/science.aat0971. PMC 6413494. PMID 30792304.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.