Letermovir
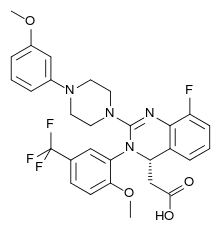
Letermovir (INN; trade name Prevymis) is an antiviral drug for the treatment of cytomegalovirus (CMV) infections. It has been tested in CMV infected patients with allogeneic stem cell transplants and may also be useful for other patients with a compromised immune system such as those with organ transplants or HIV infections.[1] The drug was developed by Merck & Co., Inc as investigative compound MK-8228.[2]
 | |
| Clinical data | |
|---|---|
| Trade names | Prevymis |
| Other names | AIC246; MK-8228 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618006 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.232.644 |
| Chemical and physical data | |
| Formula | C29H28F4N4O4 |
| Molar mass | 572.561 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
The drug was granted fast track status by the US Food and Drug Administration (FDA) and orphan drug status by the European Medicines Agency.[1] In the United States, it is approved for prophylaxis of CMV infection and disease in adult CMV-seropositive recipients of an allogeneic hematopoietic stem cell transplant.[3]
The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[4]
References
- "Neues Virostatikum Letermovir" (in German). Deutsche Apothekerzeitung. 2011-08-29.
- Masangkay, Estel Grace (July 29, 2014). "Merck Kicks Off Phase 3 Study Of CMV Drug Letermovir". Retrieved 8 Oct 2014.
- "FDA Approves Letermovir for CMV Prophylaxis Post-Transplantation". onclive.com. November 9, 2017.
- New Drug Therapy Approvals 2017 (PDF). U.S. Food and Drug Administration (FDA) (Report). January 2018. Retrieved 16 September 2020.
External links
- "Letermovir". Drug Information Portal. U.S. National Library of Medicine.
- "Letermovir Injection". MedlinePlus.