MK-434
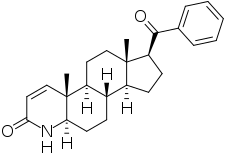
MK-434 is a 5α-reductase inhibitor which was under development in the 1990s by Merck & Co for the treatment of a variety of androgen-dependent conditions including benign prostatic hyperplasia, prostate cancer, pattern hair loss, excessive hair growth, acne, and seborrhea but was never marketed.[1][3][2] It acts as a selective inhibitor of 5α-reductase type 2.[2][3][4] The drug has been found to decrease circulating dihydrotestosterone levels by a maximum of approximately 50% in men.[2][3] MK-434 is a synthetic 4-azasteroid and is structurally related to other 5α-reductase inhibitors like finasteride.[3]
 | |
| Clinical data | |
|---|---|
| Other names | MK-0434; 17β-Benzoyl-4-aza-5α-androst-1-en-3-one |
| Routes of administration | By mouth[1][2] |
| Drug class | 5α-Reductase inhibitor |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C25H31NO2 |
| Molar mass | 377.528 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
See also
References
- https://adisinsight.springer.com/drugs/800005411
- Van Hecken A, Depré M, Schwartz JI, Tjandramaga TB, Winchell GA, De Lepeleire I, Ng J, De Schepper PJ (1994). "Plasma concentrations and effect on testosterone metabolism after single doses of MK-0434, a steroid 5 alpha-reductase inhibitor, in healthy subjects". Eur. J. Clin. Pharmacol. 46 (2): 123–6. doi:10.1007/bf00199874. PMID 8039530. S2CID 22756609.
- Chen W, Zouboulis CC, Orfanos CE (1996). "The 5 alpha-reductase system and its inhibitors. Recent development and its perspective in treating androgen-dependent skin disorders". Dermatology (Basel). 193 (3): 177–84. doi:10.1159/000246242. PMID 8944337.
- Cooke GM, Pothier F, Murphy BD (March 1997). "The effects of progesterone, 4,16-androstadien-3-one and MK-434 on the kinetics of pig testis microsomal testosterone-4-ene-5alpha-reductase activity". J. Steroid Biochem. Mol. Biol. 60 (5–6): 353–9. doi:10.1016/s0960-0760(96)00223-3. PMID 9219928. S2CID 25760027.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.