Maltol
Maltol is a naturally occurring organic compound that is used primarily as a flavor enhancer. It is found in the bark of larch tree, in pine needles, and in roasted malt (from which it gets its name). It is a white crystalline powder that is soluble in hot water, chloroform, and other polar solvents. Because it has the odor of cotton candy and caramel, maltol is used to impart a sweet aroma to fragrances. Maltol's sweetness adds to the odor of freshly baked bread, and is used as a flavor enhancer (INS number 636) in breads and cakes.
 | |
| Names | |
|---|---|
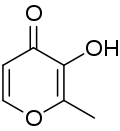
| IUPAC name
3-Hydroxy-2-methyl-4H-pyran-4-one | |
| Other names
Larixinic acid; Palatone; Veltol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.884 |
| E number | E636 (flavour enhancer) |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H6O3 | |
| Molar mass | 126.111 g·mol−1 |
| Density | 1.348 g/cm3 |
| Melting point | 161 to 162 °C (322 to 324 °F; 434 to 435 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Maltol, like related 3-hydroxy-4-pyrones such as kojic acid, binds to hard metal centers such as Fe3+, Ga3+, Al3+, and VO2+.[1] Related to this property, maltol has been reported to greatly increase aluminum uptake in the body[2] and to increase the oral bioavailability of gallium[3] and iron.[4] It is known in the European E number food additive series as E636.
Derivatives
Some synthetic derivatives of maltol, developed at the University of Urbino, showed limited in vitro antiproliferative activity towards cancer cells lines, perhaps inducing apoptosis in these cells.[5][6]
References
- B. D. Liboiron; K. H. Thompson; G. R. Hanson; E. Lam; N. Aebischer; C. Orvig (2005). "New Insights into the Interactions of Serum Proteins with Bis(maltolato)oxovanadium(IV): Transport and Biotransformation of Insulin-Enhancing Vanadium Pharmaceuticals". J. Am. Chem. Soc. 127 (14): 5104–5115. doi:10.1021/ja043944n. PMID 15810845.
- N. Kaneko; H. Yasui; J. Takada; K. Suzuki; H. Sakurai (2004). "Orally administrated aluminum-maltolate complex enhances oxidative stress in the organs of mice". J. Inorg. Biochem. 98 (12): 2022–2031. doi:10.1016/j.jinorgbio.2004.09.008. PMID 15541491.
- L. R. Bernstein; T. Tanner; C. Godfrey; B. Noll (2000). "Chemistry and pharmacokinetics of gallium maltolate, a compound with high oral gallium bioavailability". Metal-Based Drugs. 7 (1): 33–48. doi:10.1155/MBD.2000.33. PMC 2365198. PMID 18475921.
- D.M. Reffitt; T.J. Burden; P.T. Seed; J. Wood J; R.P. Thompson; J.J. Powell (2000). "Assessment of iron absorption from ferric trimaltol". Ann. Clin. Biochem. 37 (4): 457–66. doi:10.1258/0004563001899645. PMID 10902861.
- Amatori, G.Ambrosi; Fanelli, M.Formica; Fusi, L.Giorgi; Macedi, M.Micheloni; Paoli, R.Pontellini (2012). "Synthesis, basicity, structural characterization, and biochemical properties of two [(3-hydroxy-4-pyron-2-yl)methyl]amine derivatives showing antineoplastic features". J. Org. Chem. 77 (5): 2207–18. doi:10.1021/jo202270j. PMID 22296279.
- Amatori, I.Bagaloni; Macedi, M.Formica; Giorgi, V.Fusi (2010). "Malten, a new synthetic molecule showing in vitro antiproliferative activity against tumour cells and induction of complex DNA structural alterations". Br. J. Cancer. 103 (2): 239–48. doi:10.1038/sj.bjc.6605745. PMC 2906739. PMID 20571494.