Methyl orange
Methyl orange is a pH indicator frequently used in titration because of its clear and distinct color variance at different pH values. Methyl orange shows red color in acidic medium and yellow color in basic medium. Because it changes color at the pKa of a mid strength acid, it is usually used in titration for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of color change, but it has a sharp end point. In a solution becoming less acidic, methyl orange changes from red to orange and, finally, to yellow—with the reverse process occurring in a solution of increasing acidity.
 | |
 | |
 | |
| Names | |
|---|---|
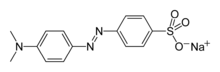
| Preferred IUPAC name
Sodium 4-{[4-(dimethylamino)phenyl]diazenyl}benzene-1-sulfonate | |
| Other names
Sodium 4-[(4-dimethylamino)phenylazo]benzenesulfonate | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.008.115 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 3143 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H14N3NaO3S | |
| Molar mass | 327.33 g·mol−1 |
| Appearance | Orange or yellow solid[1] |
| Density | 1.28 g/cm3 |
| Melting point | > 300 °C (572 °F; 573 K) (not precisely defined) |
| Boiling point | Decomposes[1] |
| 5 g/L (20 °C) | |
| Solubility in diethyl ether | Insoluble[2] |
| Hazards | |
| Main hazards | Toxic (T) |
| GHS pictograms |  |
| GHS Signal word | Danger |
| H301 | |
| P308, P310 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
60 mg/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Indicator colors

In a solution that decreases in acidity, methyl orange moves from the colour red to orange and finally to yellow with the opposite occurring for a solution increasing in acidity.
| Methyl orange (pH indicator) | ||
| below pH 3.1 | above pH 4.4 | |
| 3.1 | ⇌ | 4.4 |
In an acid, it is reddish and in alkali, it is yellow. Methyl orange has a pKa of 3.47 in water at 25 °C (77 °F).[3]
Other indicators
| Methyl orange in xylene cyanol solution (pH indicator) | ||
| below pH 3.2 | above pH 4.2 | |
| 3.2 | ⇌ | 4.2 |
Modified (or screened) methyl orange, an indicator consisting of a solution of methyl orange and xylene cyanol, changes from grey-violet to green as the solution becomes more basic.
References
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 3.384. ISBN 9781498754293.
- MSDS Archived 2014-05-12 at the Wayback Machine from ScienceLab.com, Inc. Retrieved 2011-09-24
- Sandberg, Richard G.; Henderson, Gary H.; White, Robert D.; Eyring, Edward M. (1972). "Kinetics of acid dissociation-ion recombination of aqueous methyl orange". The Journal of Physical Chemistry. 76 (26): 4023–4025. doi:10.1021/j100670a024.
External links
| Wikimedia Commons has media related to Methyl orange. |
