MicA RNA
The MicA RNA (also known as SraD) is a small non-coding RNA that was discovered in E. coli during a large scale screen.[1] Expression of SraD is highly abundant in stationary phase, but low levels could be detected in exponentially growing cells as well.
| SraD RNA | |
|---|---|
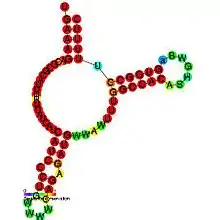 Predicted secondary structure and sequence conservation of SraD | |
| Identifiers | |
| Symbol | SraD |
| Alt. Symbols | sraD |
| Rfam | RF00078 |
| Other data | |
| RNA type | Gene; sRNA |
| Domain(s) | Bacteria |
| SO | SO:0000655 |
| PDB structures | PDBe |
Function
This RNA binds the Hfq protein and regulates levels of gene expression by an antisense mechanism. It is known to target the OmpA gene in E. coli and occludes the ribosome binding site.[2] Under conditions of envelope stress, micA transcription is induced. MicA, RybB RNA and MicL RNA transcription is under the control of the sigma factor sigma(E).[3][4][5] In E.coli, SraD also interacts in cis and trans with the mRNA species, luxS, ompA and phoP, respectively. This observation describes MicA to be the first known sRNA to carry out antisense regulation in both structural configurations. MicA is known to interact with the mRNA encoding the quorum sensing synthase homolog, LuxS in E.coli and both RNAs are processed by the double stranded RNA endonuclease, RNase III. [6] Based on its conservation, this is presumably the case in close relatives and may serve as a long elusive link between envelope stress and quorum sensing.
The PhoPQ two-component system is repressed by MicA. The RNA presumably pairs with the ribosomal binding site of phoP mRNA, thereby inhibiting translation. This links micA to cellular processes such as Mg(2+) transport, virulence, LPS modifications and resistance to antimicrobial peptides.[7][8]
In S. typhimurium MicA has been shown to be involved in biofilm formation. It is observed that the luxS mutant biofilm formation process is found to be dependent on MicA in cases where the gene's coding region is deleted, however, it is still not known to what extent MicA is involved in this mechanism. [9]
Site directed mutagensis has been used to construct mutated forms of MicA forms in order to investigate the RNA determinants important to its stability and function.[10] Each 'domain' investigated ( 5′linear domain, the structured region with two stem-loops, the A/U-rich sequence and the 3′ poly(U) tail) was altered without affecting the overall secondary structure of MicA however, each 'domain' was found to have a different impact on stability and the ability of MicA to regulate its multiple targets.[10]
References
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S (June 2001). "Novel small RNA-encoding genes in the intergenic regions of Escherichia coli". Current Biology. 11 (12): 941–50. doi:10.1016/S0960-9822(01)00270-6. PMID 11448770.
- Udekwu KI, Darfeuille F, Vogel J, Reimegård J, Holmqvist E, Wagner EG (October 2005). "Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA". Genes & Development. 19 (19): 2355–66. doi:10.1101/gad.354405. PMC 1240044. PMID 16204185.
- Udekwu KI, Wagner EG (2007). "Sigma E controls biogenesis of the antisense RNA MicA". Nucleic Acids Research. 35 (4): 1279–88. doi:10.1093/nar/gkl1154. PMC 1851643. PMID 17267407.
- Johansen J, Rasmussen AA, Overgaard M, Valentin-Hansen P (November 2006). "Conserved small non-coding RNAs that belong to the sigmaE regulon: role in down-regulation of outer membrane proteins". Journal of Molecular Biology. 364 (1): 1–8. doi:10.1016/j.jmb.2006.09.004. PMID 17007876.
- Guo MS, Updegrove TB, Gogol EB, Shabalina SA, Gross CA, Storz G (July 2014). "MicL, a new σE-dependent sRNA, combats envelope stress by repressing synthesis of Lpp, the major outer membrane lipoprotein". Genes & Development. 28 (14): 1620–34. doi:10.1101/gad.243485.114. PMC 4102768. PMID 25030700.
- Udekwu KI (October 2010). "Transcriptional and post-transcriptional regulation of the Escherichia coli luxS mRNA; involvement of the sRNA MicA". PLOS ONE. 5 (10): e13449. Bibcode:2010PLoSO...513449U. doi:10.1371/journal.pone.0013449. PMC 2956633. PMID 20976191.
- Coornaert A, Lu A, Mandin P, Springer M, Gottesman S, Guillier M (April 2010). "MicA sRNA links the PhoP regulon to cell envelope stress". Molecular Microbiology. 76 (2): 467–79. doi:10.1111/j.1365-2958.2010.07115.x. PMC 2925231. PMID 20345657.
- Coornaert A, Chiaruttini C, Springer M, Guillier M (January 2013). "Post-transcriptional control of the Escherichia coli PhoQ-PhoP two-component system by multiple sRNAs involves a novel pairing region of GcvB". PLOS Genetics. 9 (1): e1003156. doi:10.1371/journal.pgen.1003156. PMC 3536696. PMID 23300478.
- Kint G, De Coster D, Marchal K, Vanderleyden J, De Keersmaecker SC (November 2010). "The small regulatory RNA molecule MicA is involved in Salmonella enterica serovar Typhimurium biofilm formation". BMC Microbiology. 10: 276. doi:10.1186/1471-2180-10-276. PMC 2987988. PMID 21044338.
- Andrade JM, Pobre V, Arraiano CM (2013). Sumby P (ed.). "Small RNA modules confer different stabilities and interact differently with multiple targets". PLOS ONE. 8 (1): e52866. Bibcode:2013PLoSO...852866A. doi:10.1371/journal.pone.0052866. PMC 3551931. PMID 23349691.
Further reading
- Karavolos MH, Bulmer DM, Spencer H, Rampioni G, Schmalen I, Baker S, et al. (March 2011). "Salmonella Typhi sense host neuroendocrine stress hormones and release the toxin haemolysin E". EMBO Reports. 12 (3): 252–8. doi:10.1038/embor.2011.4. PMC 3059909. PMID 21331094.
- Moores A, Chipper-Keating S, Sun L, McVicker G, Wales L, Gashi K, Blomfield IC (January 2014). "RfaH suppresses small RNA MicA inhibition of fimB expression in Escherichia coli K-12". Journal of Bacteriology. 196 (1): 148–56. doi:10.1128/JB.00912-13. PMC 3911127. PMID 24163336.