Morindin
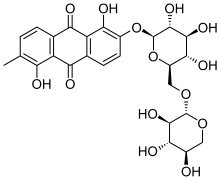
Morindin is an anthraquinone glycoside present in several Morinda species, especially M. tinctoria (the Indian mulberry tree) and M. citrifolia (noni). Chemical or enzymatic hydrolysis of morindin yields its bright red aglycone, morindone.
 | |
| Names | |
|---|---|
| IUPAC name
1,5-dihydroxy-2-methyl-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[(2S,3R,4S,5R)-3,4,5-trihydroxyoxan-2-yl]oxymethyl}oxan-2-yl]oxyanthracene-9,10-dione | |
| Other names
Morindone 6-beta-primeveroside | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H28O14 | |
| Molar mass | 564.5 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The structure and formula of morindin were first elucidated by Thomas Edward Thorpe and T. H. Greenall in 1887.[1][2]
References
- Simonsen, John Lionel (1918). "LXVI.—Morindone". J. Chem. Soc., Trans. 113: 766–774. doi:10.1039/CT9181300766. ISSN 0368-1645.
- Thorpe, T. E.; Greenall, T. H. (1887). "VI.—On morindin and morindon". J. Chem. Soc., Trans. 51: 52–58. doi:10.1039/CT8875100052. ISSN 0368-1645.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.