N-Benzoyl-N'-phenylurea
N-Benzoyl-N′-phenylurea is an organic compound with PhCONHCONHPh formula. It is benzoylurea derivative substituted with phenyl group on the opposite nitrogen atom.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
N-(Phenylcarbamoyl)benzamide | |
| Other names
1-Benzoyl-3-phenylurea | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H12N2O2 | |
| Molar mass | 240.262 g·mol−1 |
| Appearance | White crystals |
| Density | 1.259 g cm−3 |
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) |
Refractive index (nD) |
1.644 |
| Structure | |
| monoclinic | |
| P21/c, No. 14 | |
a = 15.5641(8) Å, b = 4.6564(3) Å, c = 21.1029(15) Å α = 90°, β = 128.716°, γ = 90° | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Structure and bonding
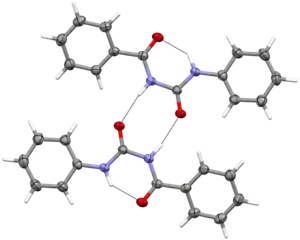
Structure of N-benzoyl-N′-phenylurea was first determined in 2010.[1] Molecules in this compound are approximately flat and exhibit high charge delocalization. Within the molecule an intramolecular N−H⋅⋅⋅O hydrogen bond is present forming pseudoaromatic 6-membered ring.[2] Additionally intermolecular N−H⋅⋅⋅O hydrogen bonds are also present combining two molecules into a centrosymmetric dimer (8-membered ring is formed).
Carbonyl C=O bond distances are equal to ca. 1.23 Å, C−N distances are in range of 1.34 to 1.41 Å.
Synthesis
In 1965 N-benzoyl-N′-phenylurea was synthesized when dry N-chlorobenzamide was reacted with phenylisocyanate or refluxed in dry benzene with anhydrous potassium fluoride.[3] Alternatively N-benzoyl-N′-phenylurea was synthesized in 2010 by hydrolysis of N-benzoyl-N′-phenylthiourea.[1]
References
- Okuniewski, Andrzej; Chojnacki, Jarosław; Becker, Barbara (2010). "N-Benzoyl-N′-phenylurea". Acta Crystallogr. E. 66 (2): o414. doi:10.1107/S1600536810001807. PMC 2979815. PMID 21579832.
- Karabıyık, Hasan; Karabıyık, Hande; İskeleli, Nazan (2012). "Hydrogen-bridged chelate ring-assisted π-stacking interactions". Acta Crystallogr. B. 68 (1): 71–79. doi:10.1107/S0108768111052608. PMID 22267560.
- Rand, Leon; Dolinski, Richard (1965). "Reactions Catalyzed By Potassium Fluoride. IV. The Reaction of N-Chlorobenzamide with Potassium Fluoride". J. Org. Chem. 30 (1): 48–49. doi:10.1021/jo01012a010.