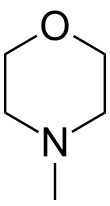
N-Methylmorpholine
N-Methylmorpholine is the organic compound with the formula O(CH2CH2)2NCH3. It is a colorless liquid. It is a cyclic tertiary amine. It is used as a base catalyst for generation of polyurethanes and other reactions. It is produced by the reaction of methylamine and diethylene glycol as well as by the hydrogenolysis of N-formylmorpholine.[2] It is the precursor to N-methylmorpholine N-oxide, a commercially important oxidant.
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
4-Methylmorpholine | |||
| Identifiers | |||
3D model (JSmol) |
|||
| Abbreviations | NMM | ||
| ChemSpider | |||
| ECHA InfoCard | 100.003.310 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C5H11NO | |||
| Molar mass | 101.149 g·mol−1 | ||
| Appearance | Liquid | ||
| Density | 0.92 g/cm3 | ||
| Melting point | −66 °C (−87 °F; 207 K) | ||
| Boiling point | 115 to 116 °C (239 to 241 °F; 388 to 389 K) | ||
| Acidity (pKa) | 7.38 (for the conjugate acid) (H2O)[1] | ||
| Hazards | |||
EU classification (DSD) (outdated) |
Flammable (F), Corrosive (C) | ||
| R-phrases (outdated) | R11 R20/21/22 R34 | ||
| S-phrases (outdated) | S16 S26 S36/37/39 S45 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
References
- David Evans Research Group Archived 2012-01-21 at the Wayback Machine
- Karsten Eller, Erhard Henkes, Roland Rossbacher, Hartmut Höke (2005). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.CS1 maint: uses authors parameter (link)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.