Octaazacubane
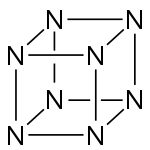
Octaazacubane is a hypothetical explosive allotrope of nitrogen with formula N8, whose molecules have eight atoms arranged into a cube. (By comparison, nitrogen usually occurs as the diatomic molecule N2.) It can be regarded as a cubane-type cluster, where all eight corners are nitrogen atoms bonded along the edges.[2] It is predicted to be a metastable molecule, in which despite the thermodynamic instability caused by bond strain, and the high energy of the N–N single bonds, the molecule remains kinetically stable for reasons of orbital symmetry.[3]
 | |
 | |
| Names | |
|---|---|
| Other names
Octaazapentacyclo[4.2.0.02,5.03,8.04,7]octane; Cubaazane; Nitrogen octaatomic molecule | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| N8 | |
| Molar mass | 112.056 g·mol−1 |
| Density | 2.69 g/cm3 (predicted)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Explosive and fuel
Octaazacubane is predicted to have an energy density (assuming decomposition into N2) of 22.9 MJ/kg,[4] which is over 5 times the standard value of TNT. It has therefore been proposed (along with other exotic nitrogen allotropes) as an explosive, and as a component of high performance rocket fuel. Its velocity of detonation is predicted to be 15,000 m/s, much (48.5%) more than octanitrocubane, the fastest known nonnuclear explosive.[1]
A prediction for Cubic gauche nitrogen energy density is 33 MJ/kg, exceeding octaazacubane by 44%,[5] though a more recent one is of 10.22 MJ/kg, making it less than half of octaazacubane.[6]
See also
- Tetranitrogen (Nitrogen allotrope with formula N4)
- Hexazine (Nitrogen allotrope with formula N6)
- Azidopentazole (Nitrogen allotrope with formula N8)
- Bispentazole (Nitrogen allotrope with formula N10)[7]
- Bis(pentazolyl)diazene (Nitrogen allotrope with formula N12)
- Eicosaazadodecahedrane (Nitrogen allotrope with formula N20)[8]
- Hexacontaazabuckminsterfullerene (Nitrogen allotrope with formula N60)[9][10]
- Pentazole
- 1,1′-Azobis-1,2,3-triazole
References
- Agrawal, Jai Prakash (2010). High Energy Materials: Propellants, Explosives and Pyrotechnics. Wiley-VCH. p. 498. ISBN 978-3-527-62880-3.
- B. Muir. "Cubane"(See under "further topics" section.)
- Patil, Ujwala N.; Dhumal, Nilesh R.; Gejji, Shridhar P. (2004). "Theoretical studies on the molecular electron densities and electrostatic potentials in azacubanes". Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta). 112: 27–32. doi:10.1007/s00214-004-0551-2. S2CID 97322279.
- Glukhovtsev, Mikhail N.; Jiao, Haijun; Schleyer, Paul von Ragué. "Besides N2, What Is the Most Stable Molecule Composed Only of Nitrogen Atoms?". Inorganic Chemistry. 35: 7124–7133. doi:10.1021/ic9606237. PMID 11666896.
- Yoo, Choong-Shik (February 2003). "Novel Functional Extended Solids at Extreme Conditions". DTIC. p. 11. Retrieved 5 October 2015.
- Bondarchuk, Sergey V.; Minaev, Boris F. (2017). "Super high-energy density single-bonded trigonal nitrogen allotrope—a chemical twin of the cubic gauche form of nitrogen". Physical Chemistry Chemical Physics. The Royal Society of Chemistry (9).
- Manaa, M. R. (2000). "Toward new energy-rich molecular systems: From N10 to N60". Chemical Physics Letters. 331 (2–4): 262–268. doi:10.1016/S0009-2614(00)01164-7.
- Charkin, O. P. (2013). "Theoretical study of N20, C20, and B20 clusters "squeezed" inside icosahedral C80 and He80 cages". Russian Journal of Inorganic Chemistry. 58: 46–55. doi:10.1134/S0036023613010038. S2CID 97510177.
- Wang, L. J.; Zgierski, M. Z. (2003). "Super-high energy-rich nitrogen cluster N60". Chemical Physics Letters. 376 (5–6): 698. doi:10.1016/S0009-2614(03)01058-3.
- "Archived copy". Archived from the original on 2013-03-03. Retrieved 2014-05-24.CS1 maint: archived copy as title (link)
External links
 Media related to Octaazacubane at Wikimedia Commons
Media related to Octaazacubane at Wikimedia Commons