Oxidosqualene cyclase
Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.[1]
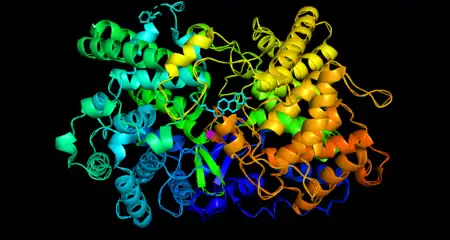
Introduction
There are two major groups of sterol-producing OSC enzymes:
- Cycloartenol synthase (CAS), found in all plants, which produces primarily cycloartenol
- Lanosterol synthase (LAS), found in all animals and fungi, and occasionally in plants, which produces primarily lanosterol
Sterols and triterpenes are extremely diverse classes of natural products, particularly in plants, which often contain numerous OSC enzymes with different substrate and product specificities;[1] common examples include lupeol synthase and beta-amyrin synthase.[2] OSC enzymes' catalytic mechanism is similar to the prokaryotic squalene-hopene cyclase.[3]
Directed evolution and protein design have been used to identify small numbers of point mutations that alter the product specificities of OSC enzymes, most notably in altering a cycloartenol synthase to produce predominantly lanosterol.[4]
Structure
Oxidosqualene cyclase is a monomeric enzyme. Its active site consists of a depression between two barrel domains.[5] The active site is mostly made up of acidic amino acids in the majority of organisms.[6] The residues in the active site make it energetically favorable for oxidosqualene to take on a more folded conformation, which closely resembles its product.[5] This crucially sets the substrate up for the series of reactions that form the rings. Oxidosqualene is located in the cell’s microsome membranes where it can easily harvest its hydrophobic substrate and turn out its hydrophobic product.[7]
Biological Function
Oxidosqualene cyclase is a key enzyme in the cholesterol biosynthesis pathway. It catalyzes the formation of lanosterol, which is then converted through many steps into cholesterol. The body uses cholesterol for temperature regulation. It is also a precursor for testosterone in males and oestradiol in females.[8]
Regulation
The enzyme’s genetic expression is regulated by sterol regulatory element binding protein (SREBP-2), a molecule which also regulates the expression of other enzymes in the cholesterol biosynthesis pathway.[9]
Enzyme Mechanism
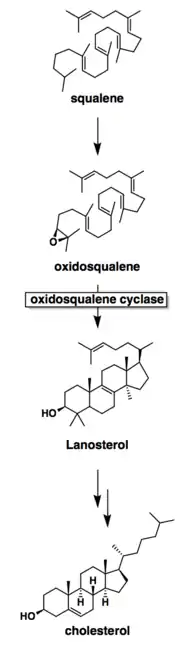
Mechanistically, the enzyme catalyzes the formation of four rings along the long chain of the substrate (oxidosqualene), producing lanosterol. This cyclization is one of the most complex known enzyme functions[10] and is highly selective.[11] In the enzyme’s active site, a histidine residue activates an aspartic acid residue, which protonates the substrate’s epoxide, setting off a series of carbon-carbon bond formations that form rings.[7][12] Finally, the enzyme deprotonates to yield lanosterol, which has a hydroxyl group instead of an epoxide. This hydroxyl group can be seen in the image above.
Disease Relevance
High blood cholesterol, also called hypercholesterolemia, significantly increases the risk of stroke, heart attack, and peripheral artery disease. If untreated, it can also lead to plaque accumulation in blood vessels, which is known as atherosclerosis.[13] For this reason, the sterol biosynthetic pathway has long been a target for the drug development industry. Statins, which inhibit HMG-CoA reductase (an enzyme that catalyzes an earlier step in the cholesterol biosynthesis pathway) are commonly prescribed to treat high cholesterol. However, the efficacy and safety of statins has been recently scrutinized in a number of reports.[14][15][16] This is largely because blocking the cholesterol biosynthesis pathway before squalene has been found to disrupt the synthesis of isoprenoids, which are used for the biosynthesis of key molecules in RNA transcription, ATP synthesis, and other essential cell activities.[17] Oxidosqualene cyclase, which is downstream of squalene in the pathway, is an attractive target for inhibition. Many inhibitors have been proposed, among them steroid analogs, phenol-based compounds, benzamide and carboxamide derivatives, and nitrogen-containing heterocyclic compounds. The most effective inhibitors have a hydrogen-bond acceptor at a specific distance away from a hydrophobic region.[6] Inhibitors of oxidosqualene cyclase have shown promise as antimicrobial agents as well, because they’ve been shown to kill off trypanosoma cruzi.[18][19] Trypanosoma cruzi is a parasite transmitted to people by insects, mostly in Latin America. The parasite causes a disease called Chagas disease, in which acute infections around an insect bite can lead to more serious complications, such as decreased heart, esophagus, colon, and even brain function.[20]
Evolution
Stork, et al. compared the protein sequences of C. albicans oxidosqualene cyclase with the analogous enzyme (squalene cyclase) in two different bacteria and found conserved regions in the former.[21] Rabelo et al. found a conserved active site across seven organisms.[6] It is believed that animal and fungal oxidosqualene cyclases likely evolved from their prokaryotic counterparts.[21]
References
- Thimmappa, Ramesha; Geisler, Katrin; Louveau, Thomas; O'Maille, Paul; Osbourn, Anne (29 April 2014). "Triterpene Biosynthesis in Plants". Annual Review of Plant Biology. 65 (1): 225–257. doi:10.1146/annurev-arplant-050312-120229. PMID 24498976.
- Sawai, S. (15 March 2006). "Plant Lanosterol Synthase: Divergence of the Sterol and Triterpene Biosynthetic Pathways in Eukaryotes". Plant and Cell Physiology. 47 (5): 673–677. doi:10.1093/pcp/pcj032. PMID 16531457.
- Wendt, KU; Poralla, K; Schulz, GE (19 September 1997). "Structure and function of a squalene cyclase". Science. 277 (5333): 1811–5. doi:10.1126/science.277.5333.1811. PMID 9295270.
- Lodeiro, Silvia; Schulz-Gasch, Tanja; Matsuda, Seiichi P. T. (October 2005). "Enzyme Redesign: Two Mutations Cooperate to Convert Cycloartenol Synthase into an Accurate Lanosterol Synthase". Journal of the American Chemical Society. 127 (41): 14132–14133. doi:10.1021/ja053791j. PMID 16218577.
- Huff, Murray; Telford, Dawn (13 June 2005). "Lord of the rings - the mechanism for oxidosqualene:lanosterol cyclase becomes crystal clear". Cell. 26 (7): 335–340. doi:10.1016/j.tips.2005.05.004. PMID 15951028.
- Rabelo, Vitor Von-Held; Romeiro, Nelilma; Abreu, Paula (July 2017). "Design strategies of oxidosqualene cyclase inhibitors: Targeting the sterol biosynthetic pathway". The Journal of Steroid Biochemistry and Molecular Biology. 171: 305–317. doi:10.1016/j.jsbmb.2017.05.002. PMID 28479228.
- "PDB101: Molecule of the Month: Oxidosqualene Cyclase". RCSB: PDB-101. Retrieved 2019-03-09.
- Berg JM, Tymoczko JL, Stryer L (2012). Biochemistry (7th ed.). New York: W.H. Freeman and Company. ISBN 978-1-4292-7635-1.
- Goldstein, Joseph L.; Brown, Michael S.; Park, Sahng Wook; Anderson, Norma N.; Warrington, Janet A.; Shah, Nila A.; Horton, Jay D. (2003-10-14). "Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes". Proceedings of the National Academy of Sciences. 100 (21): 12027–12032. Bibcode:2003PNAS..10012027H. doi:10.1073/pnas.1534923100. ISSN 0027-8424. PMC 218707. PMID 14512514.
- Gurr, M. I.; Harwood, J. L. (1991), Gurr, M. I.; Harwood, J. L. (eds.), "Metabolism of structural lipids", Lipid Biochemistry: An Introduction, Springer US, pp. 295–337, doi:10.1007/978-1-4615-3862-2_7, ISBN 9781461538622
- Bloch, Konrad. “The Biological Synthesis of Cholesterol.” Nobel Lecture, December 11, 1964.
- Ruf, Armin; Stihle, Martine; Hennig, Michael; Dehmlow, Henrietta; Aebi, Johannes; Benz, Jörg; D'Arcy, Brigitte; Schulz-Gasch, Tanja; Thoma, Ralf (November 2004). "Insight into steroid scaffold formation from the structure of human oxidosqualene cyclase". Nature. 432 (7013): 118–122. Bibcode:2004Natur.432..118T. doi:10.1038/nature02993. ISSN 1476-4687. PMID 15525992.
- "High Blood Cholesterol | National Heart, Lung, and Blood Institute (NHLBI)". www.nhlbi.nih.gov. Retrieved 2019-03-09.
- Wright, James M.; Jewell, Nicholas; Rosenberg, Harriet G.; Abramson, John D. (2013-10-22). "Should people at low risk of cardiovascular disease take a statin?". BMJ. 347: f6123. doi:10.1136/bmj.f6123. ISSN 1756-1833. PMID 24149819.
- Diamond, David M.; Ravnskov, Uffe (March 2015). "How statistical deception created the appearance that statins are safe and effective in primary and secondary prevention of cardiovascular disease". Expert Review of Clinical Pharmacology. 8 (2): 201–210. doi:10.1586/17512433.2015.1012494. ISSN 1751-2441. PMID 25672965.
- Pedersen, Terje R.; Tobert, Jonathan A. (1996-01-01). "Benefits and Risks of HMG-CoA Reductase Inhibitors in the Prevention of Coronary Heart Disease". Drug Safety. 14 (1): 11–24. doi:10.2165/00002018-199614010-00003. ISSN 1179-1942. PMID 8713485.
- Casey, Patrick (October 19, 2017). "Biochemistry of protein prenylation" (PDF). Journal of Lipid Research. 33: 1731–1739 – via ASBMB.
- Hinshaw, Jerald C.; Suh, Dae-Yeon; Garnier, Philippe; Buckner, Frederick S.; Eastman, Richard T.; Matsuda, Seiichi P. T.; Joubert, Bridget M.; Coppens, Isabelle; Joiner, Keith A. (2003-09-25). "Oxidosqualene cyclase inhibitors as antimicrobial agents". Journal of Medicinal Chemistry. 46 (20): 4240–4243. doi:10.1021/jm034126t. ISSN 0022-2623. PMID 13678402.
- Voorhis, Wesley C. Van; Wilson, Aaron J.; Griffin, John H.; Buckner, Frederick S. (2001-04-01). "Potent Anti-Trypanosoma cruzi Activities of Oxidosqualene Cyclase Inhibitors". Antimicrobial Agents and Chemotherapy. 45 (4): 1210–1215. doi:10.1128/AAC.45.4.1210-1215.2001. ISSN 0066-4804. PMC 90445. PMID 11257036.
- Prevention, CDC-Centers for Disease Control and (2017-05-02). "CDC - Chagas Disease - Disease". www.cdc.gov. Retrieved 2019-03-09.
- Stork, Gilbert; Burgstahler, A. W. (1955-10-01). "The Stereochemistry of Polyene Cyclization". Journal of the American Chemical Society. 77 (19): 5068–5077. doi:10.1021/ja01624a038. ISSN 0002-7863.