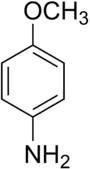
p-Anisidine
p-Anisidine (para-anisidine) is an organic compound with the formula CH3OC6H4NH2. A white solid, commercial samples can appear grey-brown owing to air oxidation. It is one of three isomers of anisidine, methoxy-containing anilines. It is prepared by reduction of 4-nitroanisole.[6]
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Methoxyaniline[1] | |||
| Other names
para-Anisidine; 4-Aminoanisole; p-Aminoanisole | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.959 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2431 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties[2] | |||
| C7H9NO | |||
| Molar mass | 123.155 g·mol−1 | ||
| Appearance | Light reddish brown solid[3] | ||
| Odor | Fishy[3] | ||
| Density | 1.071 (at 57 °C) | ||
| Melting point | 56 to 59 °C (133 to 138 °F; 329 to 332 K) | ||
| Boiling point | 243 °C (469 °F; 516 K) | ||
| Sparingly soluble[3] | |||
| Solubility in other solvents | Soluble in ethanol, diethyl ether, acetone, benzene | ||
| Vapor pressure | 0.006 mmHg (25 °C)[4] | ||
| -80.56·10−6 cm3/mol | |||
Refractive index (nD) |
1.5559 | ||
| Hazards | |||
| GHS pictograms |    | ||
| GHS Signal word | Warning | ||
| H300, H310, H330, H350, H373, H400 | |||
| P201, P202, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301+310, P302+350, P304+340, P308+313, P310, P314, P320, P321, P322, P330, P361, P363, P391, P403+233 | |||
| Flash point | 122 °C (252 °F; 395 K) | ||
| 515 °C (959 °F; 788 K) | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose) |
2900 mg/kg (rabbit, oral) 1300 mg/kg (mouse, oral) 1400 mg/kg (rat, oral)[5] | ||
LDLo (lowest published) |
1000 mg/kg (mouse, oral)[5] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
TWA 0.5 mg/m3 [skin][4] | ||
REL (Recommended) |
TWA 0.5 mg/m3 [skin][4] | ||
IDLH (Immediate danger) |
50 mg/m3[4] | ||
| Related compounds | |||
Related compounds |
o-Anisidine m-Anisidine | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Anisidine value
p-Anisidine condenses readily with aldehydes and ketones to form Schiff bases, which absorb at 350 nm. This colorimetric reaction is used to test for the presence of oxidation products in fats and oils, an official method for detecting them by the American Oil Chemists' Society.[7] It is particularly good at detecting unsaturated aldehydes, which are the ones that are most likely to generate unacceptable flavors, making it particularly useful in food quality testing.[8][9]
Safety
p-Anisidine is a relatively toxic compound with a permissible exposure limit of 0.5 mg/m3.[3]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 669. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
The names ‘toluidine’, ‘anisidine’, and ‘phenetidine’ for which o-, m-, and p- have been used to distinguish isomers, and ‘xylidine’ for which numerical locants, such as 2,3-, have been used, are no longer recommended, nor are the corresponding prefixes ‘toluidine’, ‘anisidino’, ‘phenetidine’, and ‘xylidino’.
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-98. ISBN 0-8493-0462-8.
- "Occupational Safety and Health Guideline for Anisidine (o-, p-isomers)" (PDF).
- NIOSH Pocket Guide to Chemical Hazards. "#0035". National Institute for Occupational Safety and Health (NIOSH).
- "p-Anisidine". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- Mitchell, Stephen C.; Waring, Rosemary H. (2000). "Aminophenols". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH. doi:10.1002/14356007.a02_099. ISBN 3527306730.
- "AOCS Official Method Cd 18-90". American Oil Chemists' Society. Retrieved 26 February 2013.
- Steele, Robert (2004). Understanding and Measuring the Shelf-Life of Food. Woodhead Publishing in Food Science and Technology Series. Woodhead Publishing. p. 136. ISBN 1855737329.
- A.J. Dijkstra (2016). "Vegetable Oils: Composition and Analysis". Encyclopedia of Food and Health. pp. 357–364. doi:10.1016/B978-0-12-384947-2.00708-X. ISBN 9780123849533.
External links
- International Chemical Safety Card 0971
- NIOSH Pocket Guide to Chemical Hazards. "#0035". National Institute for Occupational Safety and Health (NIOSH).