PTC tasting
PTC tasting is a classic genetic marker in human population genetics investigations.
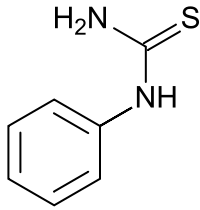
Phenyl-thio-carbamide/
Phenylthyourea
In 1931 Arthur Fox, a chemist at DuPont, in Wilmington, Delaware, synthesized phenylthiocarbamide (PTC). Some researchers reported a bitter taste when entering his laboratory, while others, including Fox himself, experienced no such sensation. Fox hypothesized that the taste was due to PTC particles suspended in the air and that some people were able to taste the chemical while others were not.
It has been suggested that the ability to taste natural chemicals similar to PTC helped human ancestors stay away from some toxic things. Substances that resemble PTC today are in some vegetables from the cabbage family (Brassicaceae) such as broccoli and Brussels sprouts.
Genetics
This variability of PTC tasting came to the attention of Albert Blakeslee at the Carnegie Institute of Genetics on Long Island, New York. Blakeslee believed that PTC tasting was genetically determined. In 1932 he published a population study that showed that PTC tasting is inherited as a dominant Mendelian trait.[1]
For seven decades, Blakeslee's genetic description of the PTC tasting was widely accepted: tasters having one or two copies of a taster allele, but non-tasters being recessive homozygotes. Then, in 2003, Dennis Drayna and his colleagues at the National Institutes of Health (NIH) cloned the gene, the bitter-tasting ability explains TAS2R38-the 38th member of the family of 2R bitter receptors.
It has been suggested that taste and smell receptors are controlled by TAS2R38, with a small intron gene of about 1000 nucleotides. It is a member of the family of G protein-coupled or 7 trans membrane cross receptors. The binding of a ligand to the extracellular region of the receptor sets an action potential that sends an impulse to the sensory cortex of the brain, where it is interpreted as a bitter taste.
This allows an experimental test for SNP at position 145 that has the highest correlation to the sample 3 polymorphisms. Students obtain isolated DNA from cheek cells by a simple salt mouthwash and amplify a region of the gene TAS2R38. The amplified fragment (amplicon) is incubated with the restriction enzyme HaeIII, comprising the SNP in their recognition sequence GGCC. HaeIII cuts the taster allele (having the sequence GGCC); this generates a length polymorphism, and the 2 alleles can be easily separated in an agarose gel.
Virtually all non-tasters (dd) cannot taste PTC, while homozygous tasters (TT) occasionally report an inability or weak ability to taste the chemical. The heterozygous genotype (Tt) has the "leakiest" phenotype as reduced or absent tasting ability is relatively common. This is formally called a heterozygous effect.
Harris – Kalmus' threshold solutions and differentiation
In 1949, Harris and Kalmus developed a method for differentiation of bimodal threshold stimuli for tasting PTC. They proposed a series of 13 solutions of these substances with serial water by halves from the initial concentration of 0.13%, so that the solution in the final test contained only a few molecules of this substance. Pure water was used as the fourteenth test liquid to provide a control. Differentiation between the two phenotypes of "tasters" and "non-tasters" occurred with the fifth solution. Then assuming that the conditional dimorphism controlled by two allele of the corresponding gene locus, the allele which controls the absence of sensitivity to taste PTC is recessive homozygote.[2][3]
- Testing solutions scale after Harris and Kalmus
| Solution | PTC (%) |
| 1 | 0.13 |
| 2 | 0.065 |
| 3 | 0.0325 |
| 4 | 0.01625 |
| 5 | 0.008125 |
| 6 | 0.0040625 |
| 7 | 0.00203125 |
| 8 | 0.001015625 |
| 9 | 0.0005078125 |
| 10 | 0.00025390625 |
| 11 | 0.000126953125 |
| 12 | 0.0000634765625 |
| 13 | 0.0000003173828125 |
| 14 | Boiled tap water was used both for making up the solutions and for controls. |
Although the view of the genetics of individual sensitivity to taste PTC changed, practically all the current data on the PTC taste (in)ability established certain of these substances originate from research by Harris and Kalmus, and such investigations are still taken. This is probably because it is not suggested a better method for mass population genetics projects.
Non-taster phenotype distribution (%) in selected populations
| Location | # of Participants | Non-taster % | References |
|---|---|---|---|
| Bosnia and Herzegovina | 7,362 | 32.02 | Hadžiselimović et al. (1982)[4] |
| Croatia | 200 | 27.5 | Grünwald, Pfeifer (1962) |
| Czech Republic | 785 | 32.7 | Kubičkova, Dvořaková (1968) |
| Denmark | 251 | 32.7 | Harrison et al. (1977)[5] |
| England | 441 | 31.5 | Harrison et al. (1977)[5] |
| Hungary | 436 | 32.2 | Forai, Bankovi (1967) |
| Italy | 1,031 | 29.19 | Floris et al. (1976) |
| Montenegro | 256 | 28.20 | Hadžiselimović et al. (1982)[4] |
| Užice, Serbia | 1,129 | 16.65 | Hadžiselimović et al. (1982)[4] |
| [Voivodina], [Serbia] | 600 | 26.3 | Božić, Gavrilović (1973) |
| Russia | 486 | 36.6 | Boyd (1950) |
| Slovenia | 126 | 37.2 | Brodar (1970) |
| Spain | 203 | 25.5 | Harrison et al. (1977)[5] |
References
- Blakeslee, Albert (Jan 1932). "Genetics of Sensory Thresholds: Taste for Phenyl Thio Carbamide". Proceedings of the National Academy of Sciences of the United States of America. 18 (1): 120–130. doi:10.1073/pnas.18.1.120. PMC 1076171. PMID 16577422.
- Harris, H.; Kalmus, H. (1949). "The measurement of taste sensitivity to phenylthiourea (PTC)". Ann. Eugen. 15: 24–31. doi:10.1111/j.1469-1809.1949.tb02419.x. PMID 15403125.
- Kalmus, H (1958). "Improvements in the classification of the taster genotypes". Ann. Hum. Genet. 22: 222–230. doi:10.1111/j.1469-1809.1958.tb01416.x. PMID 13534207.
- Hadžiselimović R., Novosel, V., Bukvić, S., Vrbić, N. (1982): Distribucija praga nadražaja za ukus feniltiokarbamida (PTC) u tri uzorka stanovništva Jugoslavije. God. Biol. inst. Univ. u Sarajevu, 35: 72-80.
- Harrison et al. (1977): Human biology – An introduction to human evolution, variation, growth and ecology. Oxford University Press, Oxford, ISBN 978-0-19-857164-3; ISBN 978-0-19-857165-0.