Phenylpyruvic acid
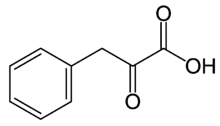
Phenylpyruvic acid is the organic compound with the formula C6H5CH2C(O)CO2H. It is a keto acid.
 | |
| Names | |
|---|---|
| IUPAC name
2-Oxo-3-phenylpropanoic acid | |
| Other names
Phenylpyruvate; 3-Phenylpyruvic acid; Keto-phenylpyruvate; beta-Phenylpyruvic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.005.317 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.160 g·mol−1 |
| Melting point | 155 °C (311 °F; 428 K) (decomposes) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Occurrence and properties
The compound exists in equilibrium with its E- and Z-enol tautomers. It is a product from the oxidative deamination of phenylalanine.
Preparation and reactions
It can be prepared by many methods. Classically it is produced from aminocinnamic acid derivatives.[1] It has been prepared by condensation of benzaldehyde and glycine derivatives to give phenylazlactone, which is then hydrolyzed with acid- or base-catalysis.[2] It can also be synthesized from benzyl chloride by double carbonylation.[3][4]
Reductive amination of phenylpyruvic acid gives phenylalanine.
References
- R. M. Herbst, D. Shemin (1939). "Phenylpyruvic Acid". Organic Syntheses. 19: 77. doi:10.15227/orgsyn.019.0077.
- Carpy, Alain J. M.; Haasbroek, Petrus P.; Oliver, Douglas W. "Phenylpyruvic acid derivatives as enzyme inhibitors: Therapeutic potential on macrophage migration inhibitory factor" Medicinal Chemistry Research 2004, volume 13, pp. 565-577.
- Wolfram, Joachim. "Preparation of α-keto-carboxylic acids from acyl halides". Google Patents US4481368 & US4481369. Ethyl Corporation.
- Werner Bertleff; Michael Roeper; Xavier Sava (2007). "Carbonylation". Ullmann's Encyclopedia of Industrial Chemistry: pg.19. doi:10.1002/14356007.a05_217.pub2. ISBN 978-3527306732.
|page(s)=has extra text (help)
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.