Sodium salts
Sodium salt are salt composed of sodium cation and the conjugate base anion of some inorganic or organic acid. They can be formed by the neutralization of the acid with sodium hydroxide.
Categorization
Sodium salts can be categorized into:
- sodium salts of carboxylic acids (e. g. sodium formate, HCOONa, the sodium salt of formic acid or sodium acetate, CH3COONa, the sodium salt of acetic acid, etc.) and
- sodium salts of inorganic acids (sulfonic acids etc.)
Organic sodium salts
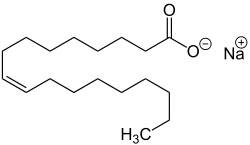
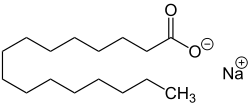
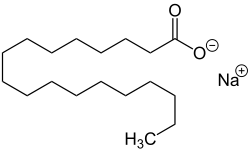
| Sodium salts of some fatty acids |
 |
 |
 |
Drugs
In pharmaceutical technology acidic pharmaceutical substances are often converted into sodium salts, because they are more stable, more soluble or membrane-permeable than the drug itself. Example of such sodium salts are (selection): Bispyribac, bithionol, bosentan, brequinar, bromfenac, Cefmenoxime, ceftiofur, citicoline, diclofenac , Floxacillin, fosinopril, naproxen, Netobimin, ozagrel, pantoprazole, pemetrexed, sitamaquin, sitaxentan, sulfamiderazin, sulfapyridine, sulfaquinoxaline, sulfathiazole, sulfazecin, thiamylal and mesna.[1] The disodium salt of cromolyn is also used as drug. Most of these salts are sodium salts of organic carboxylic acids or sulfonic acids.
Plant protection agents
Herbicides are often used as sodium salts for the reasons named above. One example is the sodium salt of methylflupyrsulfuron (CAS-No. 144740-54-5).[2]
Cosmetics
Sodium salts of long chain sulfonic acids (e.g. sodium lauryl sulfate) are often included in toothpaste and shampoo. The sodium salts of fatty acids may serve as soaps and can therefore be called sodium soaps.
Dye production
Sodium salts of certain aromatic sulfonic acids - particularly naphthalenesulfonic acid - are used in the preparation of azo dyes.
Inorganic sodium salts
Examples of important inorganic sodium salts are sodium fluoride, sodium chloride, sodium bromide, sodium iodide, sodium sulfate, sodium bicarbonate and sodium carbonate. Sodium amide (NaNH2) is the sodium salt of ammonia (NH3).
References
- The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14. Auflage, 2006, ISBN 978-0-911910-00-1.
- The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14. Auflage, 2006, S. 718, ISBN 978-0-911910-00-1.