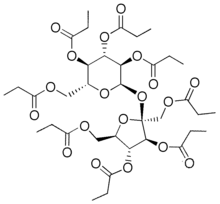
Sucrose octapropionate
Sucrose octapropionate is a chemical compound with formula C
36H
54O
19 or (C
3H
5O
2)
8(C
12H
14O
3), an eight-fold ester of sucrose and propionic acid. Its molecule can be described as that of sucrose C
12H
22O
11 with its eight hydroxyl groups HO– replaced by propionate groups H
3C–CH
2–CO
2–. It is a crystalline colorless solid.[1] It is also called sucrose octapropanoate or octapropionyl sucrose.
 | |
| Names | |
|---|---|
| IUPAC name
1,3,4,6-Tetra-O-propionyl-β-D-fructofuranosyl 2,3,4,6-tetra-O-propionyl-α-D-glucopyranoside | |
| Other names
sucrose octapropanoate, octapropionyl sucrose | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C36H54O19 | |
| Appearance | colorless crystalline solid |
| Density | 1.185 g/L [1] |
| Melting point | 45.4 °C (113.7 °F; 318.5 K) [1] |
| Boiling point | 280–290 °C (536–554 °F; 553–563 K) at 0.05 torr [2] |
| less than 0.1 g/L | |
| Solubility | ethanol, isopropanol, toluene, acetone[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
History
The preparation of sucrose octapropionate was first described in 1933 Gerald J. Cox and others.[1]
Preparation
The compound can be prepared by the reaction of sucrose with propionic anhydride in the melt state[1] or at room temperature, over several days, in anhydrous pyridine.[3]
Properties
Sucrose octapropionate is only slightly soluble in water (less than 0.1 g/L) but is soluble in many common organic solvents such as isopropanol and ethanol, from which it can be crystallized by evaporation of the solvent.[3][4]
The crystalline form melts at 45.4–45.5 °C into a viscous liquid (47.8 poises at 48.9 °C), that becomes a clear glassy solid on cooling, but easily recrystallizes.[1][3]
The density of the glassy form is 1.185 kg/L (at 20 °C). It is an optically active compound with [α]20D +53°.[3]
The compound can be vacuum distilled at 280-290 °C and 0.05 to 0.07 torr.[2]
Applications
Distillation of fully esterified propionates has been proposed as a method for the separation and identification of sugars.[2]
While the crystallinity of the pure compound prevents its use as a platicizer it was found that incompletely esterified variants (with 1 to 2 remaining hydroxyls per molecule) will not crystallize, and therefore can be considered for that application.[5]
See also
- Glucose pentaacetate
- Sucrose octaacetate
- Sucrose octabutyrate
References
- Gerald J. Cox, John H. Ferguson, and Mary L. Dodds (1933): "III. Technology of Sucrose Octaäcetate and Homologous Esters". Industrial & Engineering Chemistry, volume 25, issue 9, pages 968–970. doi:10.1021/ie50285a006
- Charles D. Hurd and R. W. Liggett (1941): "Analytical Separation of Sugars by Distillation of their Propionates". Journal of the American Chemical Society, volume 63, issue 10, pages 2659–2662.doi:10.1021/ja01855a041
- Charles D. Hurd and K. M. Gordon (1941): "Propionyl Derivatives of Sugars". Journal of the American Chemical Society, volume 63, issue 10, pages 2657–2659. doi:10.1021/ja01855a040
- Charles D. Hurd, R. W. Liggett, and K. M. Gordon (1941) "Distillation of Sugar Propionates at Low Pressures". Journal of the American Chemical Society, volume 63, issue 10, pages 2656–2657. doi:10.1021/ja01855a039
- George P Touey and Herman E Davis (1962): "Non-crystallizing sucrose lower fatty acid esters and compositions thereof". US Patent 3057743, assigned to Eastman Kodak Co.