tert-Butyldimethylsilyl chloride
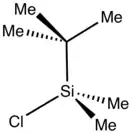
tert-Butyldimethylsilyl chloride is an organosilicon compound with the formula (Me3C)Me2SiCl (Me = CH3). It is a silane containing two methyl groups, a tert-butyl group, and a reactive chloride. It is a colorless or white solid that is soluble in many organic solvents but reacts with water and alcohols. The compound is used to protect alcohols in organic synthesis. Examples can be found in the Nicolaou taxol total synthesis.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| EC Number |
|
PubChem CID |
|
| UNII | |
| |
| |
| Properties | |
| C6H15ClSi | |
| Molar mass | 150.72 g·mol−1 |
| Appearance | white solid |
| Melting point | 86–89 °C (187–192 °F; 359–362 K) |
| Hazards | |
| GHS pictograms |   |
| GHS Signal word | Danger |
| H228, H314 | |
| P210, P240, P241, P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
tert-Butyldimethylsilyl chloride reacts with alcohols in the presence of base to give tert-butyldimethylsilyl ethers:[1]
- (Me3C)Me2SiCl + ROH → (Me3C)Me2SiOR + HCl
These silyl ethers hydrolyze much more slowly than the trimethylsilyl ethers.
Related reagents
The triflate derivative (Me3C)Me2SiOTf is used similarly but is more difficult to handle.[2]
References
- Bret E. Huff, Wenming Zhang (2008). "t‐Butyldimethylchlorosilane". EROS. doi:10.1002/047084289X.rb373.pub2.CS1 maint: uses authors parameter (link)
- "tert-Butyldimethylsilyl ethers".
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.