Trimethyl phosphite
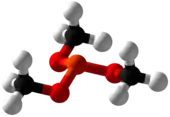
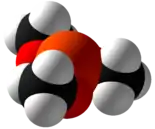
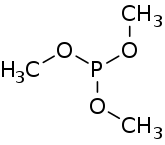
Trimethyl phosphite is an organophosphorus compound with the formula P(OCH3)3, often abbreviated P(OMe)3. It is a colorless liquid with a highly pungent odor. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis. The molecule features a pyramidal phosphorus(III) center bound to three methoxy groups.
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trimethyl phosphite[1] | |||
| Other names
Trimethoxyphosphine Trimethoxyphosphane | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.004.065 | ||
PubChem CID |
|||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C3H9O3P | |||
| Molar mass | 124.08 | ||
| Appearance | colorless liquid | ||
| Odor | distinctive, pungent[2] | ||
| Density | 1.052 | ||
| Melting point | −78 °C (−108 °F; 195 K) | ||
| Boiling point | 111 °C (232 °F; 384 K) | ||
| reacts[2] | |||
| Vapor pressure | 24 mmHg (25°C)[2] | ||
| Hazards | |||
| Flash point | 28 °C; 82 °F; 301 K [2] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible) |
none[2] | ||
REL (Recommended) |
TWA 2 ppm (10 mg/m3)[2] | ||
IDLH (Immediate danger) |
N.D.[2] | ||
| Related compounds | |||
Related compounds |
Dimethyl methylphosphonate | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Synthesis and reactions
Trimethyl phosphite is prepared from phosphorus trichloride:
- PCl3 + 3 CH3OH → P(OCH3)3 + 3 HCl
It is susceptible to oxidation to trimethyl phosphate.
It reacts with a catalytic amount of methyl iodide in the Arbuzov reaction to give dimethyl methylphosphonate:
- P(OCH3)3 → CH3P(O)(OCH3)2
As a ligand, trimethyl phosphite has a smaller cone angle and better acceptor properties relative to trimethylphosphine. A representative derivative is the colorless tetrahedral complex Ni(P(OMe)3)4 (m.p. 108 °C).[3] The tridentate ligand called the Kläui ligand is derived from trimethyl phosphite. The formation of this ligand illustrates the susceptibility of trimethyl phosphite (and metal complexes thereof) to the Arbuzov reaction.
Trimethyl phosphite is also used as a mild desulfurization reagent in organic synthesis, for example in the preparation of derivatives of tetrathiafulvalene.[4]
References
- Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 931. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- NIOSH Pocket Guide to Chemical Hazards. "#0640". National Institute for Occupational Safety and Health (NIOSH).
- Steven D. Ittel; Cushing, M. A. (1990). "Complexes of Nickel(0)". Inorganic Syntheses. Inorganic Syntheses. 28: 98–104. doi:10.1002/9780470132593.ch25. ISBN 978-0-471-52619-3.
- Larsen, Jan; Lenoir, Christine (1995). "2,2'-Bi-5,6-Dihydro-1,3-Dithiolo[4,5-b][1,4]dithiinylidene (BEDT-TTF)". Org. Synth. 72: 265. doi:10.15227/orgsyn.072.0265.
- Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_545.pub2.