Willgerodt rearrangement
The Willgerodt rearrangement or Willgerodt reaction is an organic reaction converting an aryl alkyl ketone to the corresponding amide by reaction with ammonium polysulfide, named after Conrad Willgerodt.[1][2] The formation of the corresponding carboxylic acid is a side reaction. When the alkyl group is an aliphatic chain (n typically 0 to 5), multiple reactions take place with the amide group always ending up at the terminal end.
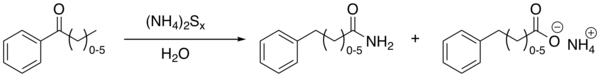
| Willgerodt rearrangement | |
|---|---|
| Named after | Conrad Willgerodt |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| RSC ontology ID | RXNO:0000185 |
An example with modified reagents (sulfur, concentrated ammonium hydroxide and pyridine) is the conversion of acetophenone to 2-phenylacetamide and phenylacetic acid[3]
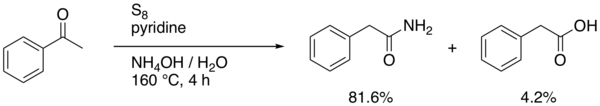
Willgerodt–Kindler reaction
| Willgerodt–Kindler reaction | |
|---|---|
| Named after | Conrad Willgerodt Karl Kindler |
| Reaction type | Rearrangement reaction |
| Identifiers | |
| Organic Chemistry Portal | willgerodt-kindler-reaction |
| RSC ontology ID | RXNO:0000186 |
The related Willgerodt–Kindler reaction[4] takes place with elemental sulfur and an amine like morpholine. The initial product is a thioacetamide for example that of acetophenone[5] which can again be hydrolyzed to the amide. The reaction is named after Karl Kindler.
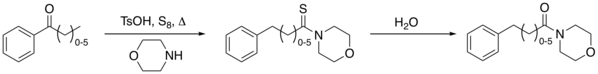
Reaction mechanism
A possible reaction mechanism for the Kindler variation[6] is depicted below:
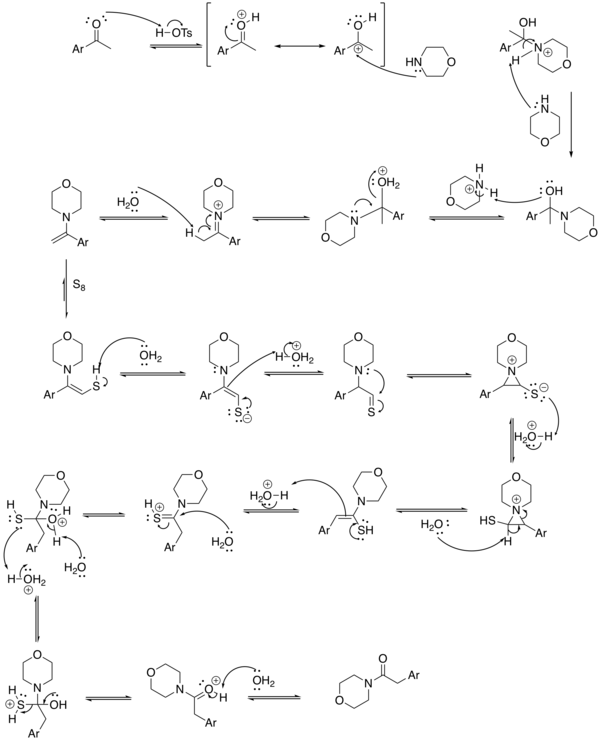
The first stage of the reaction is basic imine formation by the ketone group and the amine group of morpholine to the enamine which reacts in a conjugate addition (see Stork enamine alkylation for a related step) with sulfur to the sulfide. The actual rearrangement reaction takes place when the amine group attacks the thiocarbonyl in a nucleophilic addition temporarily forming an aziridine and the thioacetamide by tautomerization.
References
- Willgerodt, Ber., 20, 2467 (1887) doi:10.1002/cber.18870200278; 21, 534 (1888) doi:10.1002/cber.18880210195
- Carmack, M.; Spielman, M. A. Org. React. 1946, 3.
- The Willgerodt Reaction. II. A Study of Reaction Conditions with Acetophenone and Other KetonesDeLos F. DeTar and Marvin Carmack J. Am. Chem. Soc. 1946, 68(10), 2025 - 2029. (doi:10.1021/ja01214a047)
- Karl Kindler (1923). "Studien über den Mechanismus chemischer Reaktionen. Erste Abhandlung. Reduktion von Amiden und Oxydation von Aminen". Liebigs Annalen. 431 (1): 187–230. doi:10.1002/jlac.19234310111.
- Organic Syntheses, Coll. Vol. 9, p.99 (1998); Vol. 74, p.257 (1997). (Article)
- Name Reactions and Reagents in Organic Synthesis Bradford P. Mundy, Michael G. Ellerd, Frank G. Jr. Favaloro 2005 ISBN 0-471-22854-0