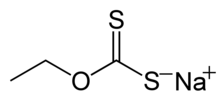
Xanthate
Xanthate usually refers to a salt with the formula ROCS−
2M+
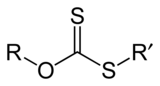
(R = alkyl; M+ = Na+, K+),[1] thus O-esters of dithiocarbonate. The name xanthates is derived from Greek ξανθός xanthos, meaning “yellowish, golden”, and indeed most xanthate salts are yellow. They were discovered and named in 1823 by Danish chemist William Christopher Zeise. These organosulfur compounds are important in two areas: the production of cellophane and related polymers from cellulose and (in mining) for extraction of certain ores.[2] They are also versatile intermediates in organic synthesis. Xanthates also refer to esters of xanthic acid. These esters have the structure ROC(=S)SR′.
_abc29.JPG.webp)
Formation and structure
Xanthate salts are produced by the treatment of an alcohol, alkali, and carbon disulfide. The process is called xanthation.[2] In chemical terminology, the alkali reacts with the alcohol to produce an alkoxide, which is the nucleophile that adds to the electrophilic carbon center in CS2.[3] Often the alkoxide is generated in situ by treating the alcohol with sodium or potassium hydroxide:
- ROH + CS2 + KOH → ROCS2K + H2O
For example, sodium ethoxide gives sodium ethyl xanthate. Many alcohols can be used in this reaction. Technical grade xanthate salts are usually of 90–95% purity. Impurities include alkali-metal sulfides, sulfates, trithiocarbonates, thiosulfates, sulfites, or carbonates as well as residual raw material such as alcohol and alkali hydroxide. These salts are available commercially as powder, granules, flakes, sticks, and solutions are available.
Some commercially important xanthate salts include:
- sodium ethyl xanthate CH3CH2OCS2Na
- potassium ethyl xanthate, CH3CH2OCS2K
- sodium isopropyl xanthate, (CH3)2CHOCS2Na
- sodium isobutyl xanthate, (CH3)2CHCH2OCS2Na
- potassium amyl xanthate, CH3(CH2)4OCS2K
The OCS2 core of xanthate salts, like that of the organic carbonates and the esters is characteristically planar. The central carbon is sp2-hybridized.
Reactions
Xanthate salts characteristically decompose in acid:
- ROCS2K + HCl → ROH + CS2 + KCl
This reaction is the reverse of the method for the preparation of the xanthate salts. The intermediate in the decomposition is the xanthic acid, ROC(S)SH, which can be isolated in certain cases.
Xanthate anions also undergo alkylation to give xanthate esters, which are generally stable:[4]
- ROCS2K + R′X → ROC(S)SR′ + KX
The C-O bond in these compounds are susceptible to cleavage by the Barton–McCombie deoxygenation, which provides a means for deoxygenation of alcohols.
They can be oxidized to the so-called dixanthogens:
- 2 ROCS2Na + Cl2 → ROC(S)S2C(S)OR + 2 NaCl
Xanthates bind to transition metal cations as bidentate ligands. The charge-neutral complexes are soluble in organic solvents.[5]
3.png.webp)
Industrial applications

Cellulose reacts with carbon disulfide (CS2) in presence of sodium hydroxide (NaOH) to produces sodium cellulose xanthate, which upon neutralization with sulfuric acid (H2SO4) gives viscose rayon or cellophane paper (Sellotape or Scotch Tape).
Certain xanthate salts and bisxanthates (e.g. Dixanthogen) are used as flotation agents in mineral processing. They are intermediates in the Chugaev elimination process and are used to control radical polymerisation under the RAFT process, also termed MADIX (macromolecular design via interchange of xanthates).
Related compounds
Rarely encountered, thioxanthates arise by the reaction of CS2 with thiolate salts. For example, sodium ethylthioxanthate has the formula C2H5SCS2Na. Dithiocarbamates are also related compounds. They arise from the reaction of a secondary amine with CS2. For example, sodium diethyldithiocarbamate has the formula (C2H5)2NCS2Na.
Environmental impacts
While biodegradable, this class of chemicals may be toxic to aquatic life at concentrations of less than 1 mg/L.[8] Water downstream of mining operations is often contaminated with xanthates.[9]
References
- IUPAC does not recommend the use of the term xanthate, although it is in current use in the scientific literature: IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Xanthate". doi:10.1351/goldbook.X06696
- Roy, Kathrin-Maria. "Xanthates". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a28_423.
- This report gives a detailed procedure for the potassium ethyl xanthate: Price, Charles C.; Stacy, Gardner W. (1948). "p-Nitrophenyl sulfide". Organic Syntheses. 28: 82. doi:10.15227/orgsyn.028.0082.
- Gagosz, Fabien; Zard, Samir Z. (1948). "A Xanthate-Transfer Approach to α-Trifluoromethylamines". Organic Syntheses. 84: 32.; Collective Volume, 11, p. 212
- Haiduc, I. (2004). "1,1-Dithiolato ligands". In McClevert, J. A.; Meyer, T. J. (eds.). Comprehensive Coordination Chemistry II. 1. p. 349–376.
- Galsbøl, F.; Schäffer, C. E. (1967). Tris (O-Ethyl Dithiocarbonato) Complexes of Tripositive Chromium, Indium, and Cobalt. Inorg. Synth. Inorganic Syntheses. 10. pp. 42–49. doi:10.1002/9780470132418.ch6. ISBN 9780470132418.
- Siegfried Hauptmann: Organische Chemie, 2. durchgesehene Auflage, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, S. 652, ISBN 3-342-00280-8.
- Besser, J.; Brumbaugh, W.; Allert, A.; Poulton, B.; Schmitt, C.; Ingersoll, C. (2009). "Ecological impacts of lead mining on Ozark streams: toxicity of sediment and pore water". Ecotoxicology and Environmental Safety. 72 (2): 516–526. doi:10.1016/j.ecoenv.2008.05.013. PMID 18603298.
- Xu, Y.; Lay, J. P.; Korte, F. (1988). "Fate and effects of xanthates in laboratory freshwater systems". Bulletin of Environmental Contamination and Toxicology. 41 (5): 683–689. doi:10.1007/BF02021019. PMID 3233367. S2CID 2696850.