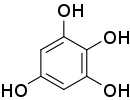
1,2,3,5-Tetrahydroxybenzene
It is a metabolite in the degradation of 3,4,5-trihydroxybenzoate (gallic acid) by Eubacterium oxidoreducens.[1]
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Benzene-1,2,3,5-tetrol | |||
| Other names
1,2,3,5-Benzenetetrol | |||
| Identifiers | |||
| |||
3D model (JSmol) |
|||
| ChEBI | |||
| ChemSpider | |||
| KEGG | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H6O4 | |||
| Molar mass | 142.110 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
1,2,3,5-Tetrahydroxybenzene is a benzenetetrol.
The enzyme pyrogallol hydroxytransferase uses 1,2,3,5-tetrahydroxybenzene and 1,2,3-trihydroxybenzene (pyrogallol), whereas its two products are 1,3,5-trihydroxybenzene (phloroglucinol) and 1,2,3,5-tetrahydroxybenzene.[2]
See also
References
- J D Haddock, and J G Ferry (1993). "Initial steps in the anaerobic degradation of 3,4,5-trihydroxybenzoate by Eubacterium oxidoreducens: characterization of mutants and role of 1,2,3,5-tetrahydroxybenzene". J. Bacteriol. 175 (3): 669–673. doi:10.1128/jb.175.3.669-673.1993. PMC 196204. PMID 8423143.
- Pyrogallol hydroxytransferase at www.uniprot.org
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.